Did you know dolphins can stay constantly awake for weeks? 25 Jun 2014 3:57 AM (10 years ago)
Young parents and workaholics are very familiar with the effects of sleep deprivation, and almost everyone is agreed – it’s not the most pleasant place to be! Anyone who has ever tried to be, or had to be, awake continuously for several days and nights will know how a stretch of busy time without a nap reduces us to clumsy, incoherent creatures in a daze. But did you know that dolphins have the incredible ability to stay constantly awake – and alert – for more than two weeks? So how do they do that? This is the question Brian Branstetter, a marine biologist at the National Marine Mammal Foundation in San Diego, has been asking. As he found out, they simply sleep with only half of their brains!Dolphins can stay awake and alert for weeks. This experiment showed they continuously echolocate and accurately report the presence of targets for at least 15 days without interruption.
In contrast to land mammals, dolphins developed the ability to sleep with only one part of their brains at a time. While half of their brains rests – and dreams – the other half remains awake and alert. This finding explains how dolphins can keep a constant lookout for their pod mates and predators, like sharks. Dolphins regularly alternate the active side of the brain. If they stopped whole brain activity and slept like humans do, they would probably become easy prey or even drown.
In a clever experiment, researchers tested how “mindful” dolphins are with just half their brain. Because dolphins use echolocation to map the world, the investigators set up a portable floating pen outfitted with eight modules, each consisting of an underwater sound projector and microphone. During echolocation, an animal produces a sound and listens to returning echoes to gain information about its environment. So when a dolphin scanned any of the eight modules using echolocation, they were able to respond to the signal with sound-mimicking echoes of signals from remote surfaces. Essentially, these modules could behave as “phantom targets.”
Trained to respond to these signals for a year, in the experiment the dolphins could eventually successfully use echolocation with extremely high accuracy and no sign of deteriorating performance for up to 15 days straight! The researchers stopped the experiments at that point. but they suggested dolphins could continue much longer staying alert and doing tasks, perhaps indefinitely. Isn’t it amazing that the dolphins showed no sign of losing their sharpness as the days wore on?
“These majestic beasts are true unwavering sentinels of the sea,” said Branstetter, according to Live Science.
Future research will include monitoring the dolphin’s brains for electrical activity via electroencephalogram, or EEG.
“Research with freely moving humans who wear portable EEG equipment has been conducted; training a dolphin to wear a similar portable EEG backpack that is capable of withstanding and functioning in an ocean environment presents much greater challenges,” Branstetter said. “However, these hurdles are not insurmountable. Also, we are interested in investigating if dolphins can perform more complex cognitive tasks without rest, like problem solving or understanding an artificial language,” Branstetter added.
If the ability to keep half the brain turned on while the other is getting a good rest is an evolutionary adaptation to protect against predators, it makes me wonder why humans didn’t also developing this ability? On the other hand, nothing is more fun than getting a good night’s sleep and bending the laws of gravity in your dreams! So for us humans it is: Keep calm and sleep on!
The study was published in PLoS One.
The Epigenetic code and brain development 21 Jan 2014 6:34 AM (11 years ago)
Epigenetics has been a hot topic in molecular biology for several years and it´s fascinating to see how it is now trending in general news as well. I was reminded of this fact when hearing Fatimah Jackson speak at the American Museum of Natural History´s recent SciCafe. So what is epigenetics? First of all it´s not as simple as the genetic code!
The name is derived from epi- (Greek: επί- over, outside of, around) combined with genetics, literally meaning being “over genetics”. Epigenetics is the study of heritable changes in gene activity which are NOT caused by changes in the DNA sequence. While the idea that factors other than changes in DNA can affect development was discussed almost a century ago (called “epigenesist” by CH Waddington), epigenetics is now more often considered to be changes to DNA that don’t involve changes in the DNA sequence itself. Moreover the term epigenetics can also be used to describe the study of stable, long-term alterations in the transcriptional potential of a cell that are not necessarily passed on to the next generation. Though this last point is contentious – some scientists believe the definition of epigenetics is modifications to DNA that are passed onto the next generation of either daughter cells (mitosis) or germ cells (meiosis).
So unlike simple genetics, where mutations affect the genotype by changing letters of the DNA alphabet (the four nitrogenous bases adenine (A), guanine (G), cytosine (C), and thymine (T)), epigenetic changes that cause changes in gene expression have other roots.
So if the DNA is not changed, what is changed instead? Generally this involves chemical modifications around DNA that cause gene expression to be changed, most often silenced. These modifications can act as an extra layer of information and, in the brain, are thought to play an important role in learning and memory, as well as in age-related cognitive decline.
The results of a study by researchers at the Salk Institute for Biological Studies published in Science show that the landscape of DNA methylation – a particular type of epigenetic modification that adds a methyl group (CH3) – is highly variable in brain cells during certain developmental stages. These new findings help us understand how information in the DNA of brain cells is controlled from early fetal development to adulthood.
With humans having exceptionally complex and large brains (larger than any other mammal in relation to body size) it is clear that building and shaping a healthy brain is the product of a long process of development. We know that the front-most part of our brain (the prefrontal cortex) for example is a critical part of the executive system, which refers to planning, reasoning, and judgment. The brain accomplishes all of this through the interaction of specialized cells such as neurons and glia, the brain’s communication specialists. We know that these cells have distinct functions.
But now epigenetics tells us what gives these cells their individual identities! It all depends on how each cell expresses the genetic code. And this how is done by epigenetic modifications, fine-tuning which genes are turned on or off without changing the DNA sequence, and thus subsequently helping to distinguishing different cell types.
The Salk scientists found that the patterns of DNA methylation undergo dynamic rearrangements in the frontal cortex of mouse and human brains during a time of development when synapses, or connections between nerve cells, grow rapidly. By comparing the exact sites of DNA methylation throughout the genome in brains from infants through adults, the researchers noticed that one form of DNA methylation can be found in neurons and glia from birth. However, a second form of DNA methylation that is almost exclusive to neurons accumulates as the brain matures, becoming the most prevalent form of DNA methylation in adult human neurons.
Teachers and child development experts have long known about natural breakage points in a child’s development. These new results can help us to understand how those points occur as the intricate DNA landscape of brain cells develops during the key stages of childhood.
What is the mechanism of DNA methylation? As mentioned above, the genetic code in DNA is made up of four nitrogenous bases A, T, C, and G. DNA methylation typically occurs at so-called “CpG sites,” where C (cytosine) sits next to G (guanine) in the DNA alphabet. Interestingly about 80–90% of CpG sites are methylated in human DNA. Moreover, in human embryonic stem cells and induced pluripotent stem cells, a type of artificially derived stem cell, DNA methylation can also occur when G does not follow C, called “non-CG methylation.” Originally, scientists thought that this type of methylation disappeared when stem cells differentiate into specific tissue-types. This latest study found this is not the case in the brain, because non-CG methylation appears after cells differentiate, and they usually differentiate during childhood and adolescence when the brain is maturing. What this finding underlines is that at the time the neural circuits of the brain mature, a parallel process of large-scale reconfiguration of the neural epigenome takes place.
The study also included the first comprehensive maps of how DNA methylation patterns change in the mouse and human brain during development (see insert). Future research can explore how changes in methylation patterns may be linked to human diseases, including psychiatric disorders like schizophrenia, depression, and bipolar disorder.
—————-
Further reading:
You can even get a quick PowerPoint podcast on the research!
https://dl.dropboxusercontent.com/u/1421283/Epigenome_Movie.mov
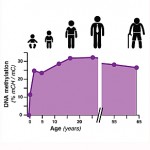
The first comprehensive maps of epigenetic changes in the brain known as "DNA methylation," a chemical modification of a cell's DNA that can act as an extra layer of information in the genome. Credit: Lister et al, 2013.
Naughty or nice? The Moral Molecule 9 Jan 2013 11:29 AM (12 years ago)

Oxytocin (ball-and-stick) bound to its carrier protein neurophysin (ribbons) based on: "Crystal structure of the neurophysin-oxytocin complex" Rose, J.P., Wu, C.K., Hsiao, C.D., Breslow, E., Wang, B.C. (1996) Nat.Struct.Biol. 3: 163-169
I recently enjoyed a truly mind-blowing talk at the New York Academy of Sciences. The Neuroeconomist (yes, he studied Economy and is founding director of Claremont´s Center for Neuroeceonomic Studies) Paul J. Zak spoke about his research on the brain chemical oxytocin (OXT) – the so-called “love hormone” – and how he showed that OXT is the source of love and prosperity, triggering a wide variety of physical and psychological effects in both women and men. In his experiments he measures OXT levels found in the blood stream of thousands of people in a variety of settings: attending a wedding, playing football, on Facebook, or play economic games in a lab. By comparing OXT levels before and after those emotionally-charged activities, he found that the level always spiked up during the activity. And interestingly, this is followed by more relaxed, trusting and caring social behavior. Oxytocin seems to be a hidden master controller of human behavior.
The hormone’s influence on our behavior and physiology originates in the brain, where it’s produced by a structure called the hypothalamus, then transferred to the pituitary gland, which releases it into the bloodstream. Like antennas picking up a signal, OXT receptors are found on the outside of cells throughout a body. As Dr. Zak showed, OXT levels tend to be higher during both stressful and socially-bonding experiences (see references for details). So how did it evolve?
Oxytocin is a peptide hormone found in almost all mammals. The present day OXT molecule evolved from a fish “fight-or-flight” molecule called vasotocin. By a random mutation, vasotocin changed one day into a two-amino acid shortened version, called isotocin. Isotocin reduced anxiety in the fish so it relaxed, which facilitated mating instead of a fight-or flight stress response. A variant of isotocin then finally became oxytocin. Similarly, vasopressin evolved into the variant arginine-vasopressin which still works in modern humans as a molecular guide towards reproductive and moral behavior. Oxytocin and vasopressin are the only hormones released by the posterior pituitary gland that can affect cells in distant parts of the body.
For a long time OXT was best known for its role in sexual reproduction, in particular during and after childbirth. But recent studies show that OXT also plays a role in ‘tribal’ behavior and trustworthiness.
Oxytocin helps our brains break down the barrier between self and others, allowing us all to practice empathy and feeling towards others. And so our brains respond to observed pain or pleasure in the same way as the pain would be happening if we were actually experiencing it; we literally experience the other person’s pain or pleasure as if it were our own.
The higher our OXT level, the closer we appear to act on the Golden Rule: You be nice to me and I´ll be nice to you. On a cellular level we need OXT-producing neurons and functioning OXT receptors in the brain. Oxytocin also directly influences the release of the two feel-good neurochemicals: dopamine and serotonin. However stress, trauma, testosterone, mental conditioning and genetic anomalies can inhibit this effect and with dropping OXT levels our moral behavior strays from the Golden Rule (though, depending on the circumstances this could be a good thing, too!).
People with chronic OXT deficiency do have altered social behavior, depending on the degree of OXT impairment. This can range from high-functioning and brilliant autism to psychopaths. Furthermore, genetic differences in the OXT receptor gene (OXTR) have been associated with maladaptive social traits, such as aggressive behavior.
The good news is that we can consciously use the “moral molecule” to make our own lives better. Oxytocin can be easily transiently boosted by a loving relationship, meditation, dance, connecting via social media and even a simple hug. Dr. Zak – who refers to himself as “Dr. Love” – told the audience I was part of to “share the love” and give a minimum of eight hugs a day! He promises that if you give eight hugs a day you´ll be happier, and the world will be a better place because you will be actively causing other people’s brains to release OXT.
Let´s hope that they are susceptible to the moral molecule and in turn will treat others more generously, causing them to release more OXT….you got the idea! The Beatles already sung it: “Love is all you need….”
————————————————-
Further reading:
Zak, Paul J. (2012), The Moral Molecule: the source of love and prosperity. Dutton, Penguin Publishing group.
Zak PJ, Stanton AA, Ahmadi S (2007). Brosnan, Sarah. ed. “Oxytocin Increases Generosity in Humans”. PLoS ONE 2 (11): e1128. doi:10.1371/journal.pone.0001128. PMC 2040517. PMID 17987115.
Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005). “Oxytocin increases trust in humans”. Nature 435 (7042): 673–6. doi:10.1038/nature03701. PMID 15931222.
Kirsch P, Esslinger C, Chen Q et al. (2005). “Oxytocin modulates neural circuitry for social cognition and fear in humans”. The Journal of Neuroscience 25 (49): 11489–93. doi:10.1523/JNEUROSCI.3984-05.2005. PMID 16339042.
Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y (2009). “Intranasal administration of oxytocin increases envy and schadenfreude (gloating)”. Biological Psychiatry 66 (9): 864–70. doi:10.1016/j.biopsych.2009.06.009. PMID 19640508.
Brain size and intelligence- why a human is smarter than a mouse 13 Nov 2012 9:23 AM (12 years ago)

“Section through the cerebral cortex of a mouse, stem cells can be seen glowing in green, mature nerve cells in red; cell nuclei for both types of cell are shown in blue.” Source: IMBA
Your brain is a complex, highly organized organ. Each mammalian brain is made of approximately 10-15 billion nerve cells, called neurons. And each brain is built of thousands of different types of neurons, called neuronal subtypes. Neurons have the amazing ability to gather and transmit electrochemical signals, the more neurons the faster signals can be transmitted; think of them like the gates and wires in a computer. It has been known that neurons arise from a small set of progenitor cells that divide in a spatially and temporally controlled manner to generate a fully functional adult cortex. However what drives daughter cells of these progenitors to different fates is poorly understood.
So the more nerve cells a brain is able to make, the smarter an organism should be. Turns out that humans are really good at it! Stem cells in the human brain produce far more nerve cells than corresponding cells in mice. Jürgen Knoblich and his research team at the Vienna Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA) found out what mechanisms are responsible, and why the orientation of the cells plays a role.
It is understood that although the genes of mice and humans are more than a 90% alike, the cerebral cortex of a mouse has around eight million neurons while in humans there are more than 10-15 billion. Nerve cells are produced in the brain of the embryo from stem cells that continuously divide. Each dividing stem cell gives rise to a nerve cell and another stem cell. So how could it be that humans have more neurons – and a much larger brain – than mice? The Knoblich laboratory suggests that it has to do with controlling the direction of cell division.
Generally spoken each stem cell can divide in different spatial planes (or directions); the daughter cells are then either ‘up and down’ or ‘left and right.’ According to current understanding the direction of division of stem cells defines whether new nerve cells, or only new stem cells, are produced. This is called a positional effect.
The IMBA scientists bred mice in which the direction of division of the stem cells can be controlled. This regulation is possible by using the protein ‘Inscuteable,’ which works like a switch for the direction of division: cells divide horizontally with Inscuteable but vertically without the protein.
Studies of the mice with Inscuteable showed that nerve cells are actually generated in both vertical and horizontal divisions (and not only in one); however the cells were far more parallel to the cell surface. So a mouse with more Inscuteable protein has more horizontal divisions, and so overall more nerve cells. A lack of Inscuteable has the opposite effect. This mechanism could be responsible for the tremendous proliferation of nerve cells in the human brain!
But how does a human brain manage to generate the correct numbers of neurons?
Higher organisms like humans reproduce nerve cells through a ‘detour,’ meaning horizontal division initially creating a stem cell and an intermediate progenitor. This cell has lost its stem cell properties but can still divide, on average once in mice, so that two nerve cells are generated per horizontal stem cell division. This indirect neurogenesis is also controlled by the Inscuteable protein.
Indirect neurogenesis seems to be the key to larger and more intelligent brains. If we compare mice to organisms with less developed brains we can see that they are lacking this kind of fast neurogenesis and have accordingly fewer nerve cells. Therefore indirect neurogenesis is a very important in terms of evolution. In humans intermediate progenitors are already much more complex and divide more frequently than in the mouse; therefore compared with mice, humans have a plethora of nerve cells.
The researchers also tried to determine whether the mice without the Inscuteable protein are dumber than their counterparts due to fewer nerve cells, or whether an artificially induced overproduction of the protein could lead to more intelligent animals, but couldn’t prove either hypothesis, yet.
So does the Inscuteable protein make man human? “Far more interesting however is the role played by Inscuteable in humans” says Jürgen Knoblich. “It probably also regulates the number of neurons in our own bodies by activating indirect neurogenesis, the evolution of the protein and its function may have contributed to the enormous enlargement of the human brain.”
This hypothesis is supported by the finding that the division pattern of the intermediate progenitors closely correlates with the level of intelligence. This specific pattern only appears in primates, including humans, so without Inscuteable we would certainly not be what we are.
References:
Mouse Inscuteable Induces Apical-Basal Spindle Orientation to Facilitate Intermediate Progenitor Generation in the Developing Neocortex Maria Pia Postiglione, Christoph Jüschke, Yunli Xie, Gerald A. Haas, Christoforos Charalambous, Juergen A. Knoblich Neuron – 20 October 2011 (Vol. 72, Issue 2, pp. 269-284)
DNA Barcoding Exposes Deregulation of Herbal Supplements 12 Oct 2012 12:54 PM (12 years ago)
 There is an increasing need for more Americans to improve their health and lifestyle. One growing trend that I see in my hometown of New York City is the building of local farms on rooftops and the increase of organic produce sections in the supermarkets. In an effort to protect our health some of us are choosing more natural and locally grown food over mass-produced, insecticide-ridden crops. And in the realm of medicine, many people are choosing more natural medicines or herbal supplements to support our health. Many common herbal medicines used today are used for a variety of reasons, from lowering cholesterol to preventing colds.
There is an increasing need for more Americans to improve their health and lifestyle. One growing trend that I see in my hometown of New York City is the building of local farms on rooftops and the increase of organic produce sections in the supermarkets. In an effort to protect our health some of us are choosing more natural and locally grown food over mass-produced, insecticide-ridden crops. And in the realm of medicine, many people are choosing more natural medicines or herbal supplements to support our health. Many common herbal medicines used today are used for a variety of reasons, from lowering cholesterol to preventing colds.
Despite the fact that the foods we eat and the medicines we take can indeed be “natural”, there are still some inherent dangers in some of them. Similar to prescription medications, some herbal supplements can cause serious side effects such as liver and lung damage, or can interfere with other medications taken at the same time. What makes herbal supplements perhaps more dangerous is the fact that they are not as highly regulated as prescription drugs. Manufacturers of herbal supplements do not need approval from the FDA in order to be put on the market. This begs the question of whether herbal supplements either have extra ingredients or are missing vital ingredients that affect their efficacy and safety.
This lack of regulation peaked the curiosity of young scientists at Hostos-Lincoln Academy in Bronx, NY. A group of high school students participated in the Urban Barcode Project (www.urbanbarcodeproject.org) and investigated gingko products sold in a variety of pharmacies and tried to identify what species of plants were primarily found in both dried leaf, liquid, and pill forms. The students found an interesting result using DNA barcoding; many of the packaged products contain mostly rice product and very little gingko. Many leaf products contained primarily Atropa belladonna, Thymus vulgaris, Salvia pratensis, and different species from the genus Nicotiana, which may be potentially harmful.
Scientists at the New York Botanical Garden (NYBG) have been working on similar projects. Damon Little, a bioinformaticist at the NYBG, investigated black cohosh, a menopause herbal supplement that has been known to be inconsistently effective in test trials. He thought these mixed results were due to the varied production of black cohosh pills. Perhaps some of these pills lack the vital ingredient for relieving menopausal symptoms, the actual black cohosh itself.
Little used DNA barcoding to identify the main species found in a variety of black cohosh pills. He discovered that as many as one quarter of the tested pills contained no black cohosh at all, but instead a related species. This can be a particularly serious problem; not only can a different species decrease the supplement’s effectiveness, but it can be potentially toxic to humans.
Unfortunately, these findings do not prove that the variety of black cohosh pills affect the variety of outcomes for users. The pills that were barcoded were purchased in New York City and online, but they were not the pills used in clinical trials. The next step would be to see if people who take pills without black cohosh are affected differently compared to those who take pills with black cohosh. It would be interesting to see if there is such a strong correlation. Perhaps with more convincing evidence, we can push for more regulation of herbal supplement production.
For more information go to:
Herbal Menopause Supplement Often Contains Other Species, DNA Barcoding Reveals
DNA Barcode Identification of Black Cohosh Herbal Dietary Supplements
Herbal supplements: What to know before you buy
Twilight’s Edward and Bella – Romance or Rabies? A scientific view on vampires 28 Sep 2012 5:32 AM (12 years ago)
Today, September 28th, is world rabies day! Rabies is an animal borne viral disease that kills nearly 100 percent of its victims once the infection reaches the brain. But have you ever wondered how this fatal virus can affect the brain, causing victims to become ´rabid´? I was thinking about it in those autumn days when all these pumpkin-spice lattes and fancy Hokkaido soups around the city indicate that Halloween is just around the corner – which is always a good excuse to enjoy classic splatter movies like Shawn of the Dead or the zombie film I Am Legend once again, or get on line to be the first to see the latest Twilight movie this November.
I am not the only scientist wondering where all this interest might come from. In 1998 the Spanish physician Juan Gòmez-Alonso proposed in the top-tier journal Neurology that scary tales of vampires and werewolves, mythologized in Hollywood movies and TV shows, may have a factual basis – originating from stories of humans infected with the rabies virus.
The parallels are quite interesting:
Both rabies and vampirism are transmitted by a bite. Both cause face spasms, hydrophobia (a fear of water) and an inability to face one’s own reflection in a mirror. Both show a hypersexualized behavior, with male rabies patients ejaculating up to 30 times per day in the final stages of infection. Finally, contrary to the Hollywood interpretation of a vampire living for centuries, the earliest mythology put the life span of a vampire at about 40 days, similar to the time is takes untreated rabies to kill a human. Gòmez-Alonso also found more evidence when examining geography and culture: geographic areas that experienced particularly devastating epidemic rabies outbreaks in the past – like the Balkans – are rich in popular folkloric myths and legends about vampires and hematofages (animals feeding on blood) with different features and behaviors.
The rabies is a virus belonging to the Rhabdoviridae family. It is a highly fatal disease that causes acute encephalitis, responsible for approximately 55,000 deaths each year worldwide, mainly in Asia and Africa. In 2010, the United States reported 6,153 animal cases of rabies across all states (including Puerto Rico) with the exception of Hawaii. Luckily, human acquisition of rabies in the US is a relatively rare occurrence, with only 2 human cases recorded for the same year (CDC data).However, the yearly mortality rate in other countries is significantly higher; for example, in India more than 25,000 people fall victim to rabies each year.
The rabies virus reproduces in both humans and animals, and is found in not only nervous tissue, but also in saliva, making the transmission of the virus easier….a bite will do. Non-animal associated transmission of rabies is extremely rare, but has occurred by means of transplant surgery.
The normal mode of transmission of this disease is by direct contact between animal and man. The animal implicated most frequently is man’s best friend – the dog, causing 99% of human rabies deaths – but other common zoonotic reservoirs (zoonosis meaning that an infectious disease can be transmitted between species) of the disease include bats, foxes, and skunks. Transmission from bats occurs through direct bites, skin-to-skin contact and through inhalation of aerosolized bat feces (like in caves with high bat populations).
The primary wild reservoir of rabies in the US is Procyon lotor, or the common raccoon; and the highest density of raccoons in New York State is…. New York City! The rabies population is under tight observation by the city health department because New York City experienced a Rabies Outbreak in 2009–2010 in New York´s favorite dog-walking destinations Central Park and Inwood Hill Park. It took one year of trapping and vaccinating animals before the numbers significantly dropped back to 1 reported case in 2012.
The rabies virus reproduces in both human and animal reservoirs, and is found in not only nervous tissue, but also in saliva, making the transmission of the virus easier….a bite will do. Transmission from bats occurs through direct bites, skin-to-skin contact and through inhalation of aerosolized bat feces (like in caves with high bat populations).
Non-animal associated transmission of rabies is extremely rare.
After a typical human infection by a dog bite, rabies replicates in muscles and spreads from the bite wound into the peripheral nervous system, moving about 1–2cm per day. It then travels along the nerves from the peripheral nervous system to the central nervous system (CNS), driven by an unknown mechanism. The period between the inoculation of the virus into the victim/host and its invasion of the CNS is the incubation period. The median incubation period is 85 days (range 40–150 days). During this phase, the virus causes quite diffuse and nonspecific symptoms within the host, including fever, sore throat, chills, malaise, anorexia, headache, nausea, vomiting, shortness of breath, cough, and weakness.
At this stage vaccination can still initiate cell-mediated immunity to prevent symptomatic rabies. But once the virus reaches the brain treatment is useless; it quickly causes encephalitis and more extreme symptoms appear. This is called the “prodromal” phase and patients die within weeks.
There are two forms of canine rabies in the prodromal phase, a “furious” (encephalitic) or “dumb” (paralytic) form. Furious rabies is characterized by high fever, hyperactivity, hypersexuality, including an increase in sexual appetite and priapism (a painful medical condition, in which the erect penis or clitoris does not return to its flaccid state) of several days, along with dysfunction of the autonomic nervous system, and abnormal looking pupils (WHO). It is this form or rabies that people think of when they hear the word “rabies,” especially as the autonomic dysfunction also includes excess salivation, producing the famous “foaming at the mouth.”
The dumb form progresses from the peripheral weakness around the transmission area to a generalized craniospinal weakness and cumulates in final encephalitis (inflammation of the brain).
On a cellular level rabies can be diagnosed prior to the appearance of symptoms by the presence of Negri bodies (inclusion bodies found in nerve cells) and a direct fluorescent antibody test (dFA). However, the dFA test requires brain tissue, and is therefore performed post-mortem. It is the test of choice for the testing of rabid animals but for living humans it is necessary to perform several other tests to diagnose rabies before death. The two main tests are PCR-based tests on saliva, or testing the blood serum or spinal fluid for antibodies. Additionally, skin biopsy specimens of hair follicles may display a rabies antigen within skin nerves.
So, happy World Rabies Day and have fun at the movies – now you can watch the films as a scientist as well as a horror fan!
———————————————–
Further reading:
Juan Gomez-Alonso; Rabies : A possible explanation for the vampire legend, Neurology 1998;51;856
Rabid: A Cultural History of the World’s Most Diabolical Virus
by Bill Wasik, Monica Murphy, Viking, www.penguin.com
Decoding ‘Mad snake disease’ 28 Aug 2012 11:58 AM (12 years ago)

A boa constrictor with IBD, "mad snake disease".
Have you ever seen a sick boa constrictor? All of a sudden they start shedding, develop head tremors and secondary infections, twisting up into knots and wasting away. These poor animals may have acquired a fatal infectious disease called inclusion body disease (IBD). The disease can rapidly progress to the nervous system, with behavioral abnormalities such as disorientation, corkscrewing of the head and neck, holding the head in unnatural positions, or rolling onto the back. Affected snakes either die quickly or starve slowly over several years. The disease was first observed in captive snakes in zoos in the mid 1970s but the cause of the disease remained elusive. Unfortunately no treatment exists; snakes diagnosed with IBD are euthanized to stop transmission to other animals.
IBD is named after large eosinophilic inclusions (or “junk” in the form of huge protein aggregates) in the cytoplasms of nearly every cell in almost all tissues, possibly caused by replication of an unknown retrovirus. However it was unclear how the virus was transmitted.
Now the riddle has been solved and IBD treatments might be possible soon. And even more than that, by investigating the origin of IBD, the Joseph L. DeRisi lab at the University of California, San Francisco, identified a virus that shares characteristics with two known virus families that can cause fatal hemorrhagic fevers in humans!
It is well-established that some of the most medically important human diseases have origins in viruses from animal populations, or have animal reservoirs. Examples include HIV-1 and -2, influenza viruses, West Nile virus, severe acute respiratory virus (SARS), coronavirus, henipaviruses, rabies viruses, hantavirus, filoviruses, and arenaviruses. Therefore animal viruses and their hosts are excellent models for studying host-pathogen interactions and vaccine development.
To computationally identify the virus the researchers used high-throughput sequencing methods to search for candidate causes of IBD. Retroviruses are RNA viruses; each snake cell already contains 95% snake RNA needed for cell viability, plus the virus RNA. But how to separate the snake RNA from the virus RNA? The scientists simply compared sequences from the infected snake to sequences from a healthy snake to figure out what was foreign and therefore might belong to the virus. The problem was that the boa constrictor genome had not yet been sequenced. DeRisi organized the “Assemblathon 2” contest, in which teams competed to develop a computer program to assemble genetic sequences in a previously unknown animal genome, preferentially the boa constrictor genome.
The result of the RNA comparison shocked the scientists.
The foreign RNA sequences that were not present in the boa constrictor genome had several similarities to arenavirus genes. These similarities revealed the cause of the illness to be a completely new set of two arenaviruses. These viruses looked like distant relatives of other arenaviruses but had protein coats that were more similar to those of Ebola viruses. While nasty arenaviruses are common in rodents and cause infections in other mammals, we were unaware that they could infect reptiles. Like arenaviruses, Ebola viruses can cause fatal hemorrhagic fever or encephalitis when transmitted to humans. Neither of those viruses had ever been known to infect reptiles, and although it had been postulated that they shared a common ancestor, no link had ever been discovered.
The next step was genome assembly (building a complete genome out of raw data) using open access bioinformatics software, and then comparison with the RNA data. They found that the sequences from the snake virus belonged to four genes—one of which was most similar to genes found in filoviruses.
Turning back to the sick snakes, the scientists found the newly identified virus in six of eight snakes with IBD, and were able to isolate the virus.
Now the team had to find a way to grow the virus so that it could be studied further. They generated Boa constrictor cell lines to perform in vitro virus culture. When the virus was introduced into healthy boa constrictor cells, the virus replicated and the cells became clogged with giant protein aggregates like those in snakes with IBD. Antibodies aimed against the virus showed that these clumps were indeed derived from arenavirus protein, further strengthening the association of this new virus and the deadly disease. A final proof of this hypothesis will be a “challenge study,” where researchers intentionally infect boa constrictors and other captive snakes with the virus in order to induce and study IBD.
IBD is a very important disease of captive snakes. In solving this longstanding veterinary mystery and enabling the first steps towards treatment, vaccines, and perhaps even eradication of this disease, these scientists also discovered an unexpected new branch of virus biology: the viruses they found appear to be a combination of arenavirus and filovirus, neither of which had been known to infect reptiles. Their existence in reptiles raises an array of important questions about host range, evolution, basic biology and emergence of new diseases associated with this poorly understood branch of viral phylogeny.
—————
Further information:
The article, “Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: a candidate etiological agent for snake inclusion body disease (IBD)” by Mark D. Stenglein, Chris Sanders, Amy L. Kistler, J. Graham Ruby, Jessica Y. Franco, Drury R. Reavill, Freeland Dunker, and Joseph L. DeRisi is published in the open access journal mBio.
Press release video:
This week in virology podcast:
http://www.twiv.tv/2012/08/19/twiv-196-an-arena-for-snakes/
Vincent Racaniello´s virology blog:
Jumping Down the Road to Cancer. 28 Aug 2012 7:21 AM (12 years ago)
Lying dormant in our genomes are millions of jumping genes. Originally discovered by Barbara McClintock, transposons are DNA sequences that can move from one location to another in our DNA. Transposons cause mutations when they jump to new locations, so keeping them from jumping is important. However, although transposons are largely silent, every person probably has a few “rare” sites, found in only a few people in the world, where a transposon has jumped to a new location.
Mutations in numerous pathways need to accumulate for cancer to progress. Given the ability of transposons to cause mutation and the role of mutation in cancer, it seemed likely that transposons would play a role in cancer. A few years ago, Iskow and colleagues showed that transposons jump in some lung tumors, suggesting a link to cancer progression. They also showed that methylation levels are often lower in lung cancers. Methylation is important for transposons silencing, so they hypothesized that lowered methylation in cancer could lead to more transposon jumps. This would “destabilize” the genome, allowing more mutations to accumulate, and accelerating cancer progression.
However, very little evidence of this connection existed until recently. With the advent of high-throughput sequencing, it is becoming possible to examine changes in the genomes of cancer cells. Lee and colleagues report on one such study. They decided to look at the effect of retrotransposons by comparing the location of these jumping genes in normal and cancer cells. Retrotransposons copy their sequence from one location to another by going through an RNA intermediate that is read “backwards” from RNA to DNA.
In their study, they had to overcome a problem: because transposons are found throughout the genome and are mostly the same in different individuals, it is hard to figure out exactly where new transposons are located. To sort this out, they developed a bioinformatics tool that could align sequence to a reference genome and identify new transposon sequence associated with this sequence. They then used normal tissue and cancer tissue from the same individual to identify transposition events in cancer cells.
Interestingly, different cancer types had different numbers of transposon jumps. Brain and blood cancers did not have many transposon-induced mutations, while epithelial cancers had frequent insertions. These jumping-gene insertions are probably important for cancer, as many of the insertions occur within genes known to affect cancer biology.
If these jumping genes cause mutations and promote cancer, why are they there? It’s still an area of contention, but all that jumping around helps provide diversity in our genomes. Sometimes that will prove to be bad, but it also allows natural selection to act on the diversity, allowing new, helpful innovations in our DNA power evolution.
Iskow el al, 2010. Natural mutagenesis of human genomes by endogenous retrotransposons. Cell. 141(7):1253-61.
Lee et. al, 2012. Landscape of Somatic Retrotransposition in Human Cancers. Science. 337(6097): 967-971.
The flavor of extinction in a bowl of soup 27 Aug 2012 9:23 AM (12 years ago)
The most expensive bowl of soup I have ever had in my life was a $15 bowl of ramen in the NYC restaurant Ippudo. And this was no ordinary “ramen” you would eat at home as a cash-strapped college student. This was an authentic bowl nuanced with so many rich and hearty flavors of Japanese cuisine.
Little did I know that $15 soup was considered “cheap” compared to other soups that sell for at least $100 a bowl. One such soup is shark fin soup, which is traditionally served in Chinese cuisine during special occasions. One is apt to eat this delicacy, not so much for its taste, but more for the gelatinous texture of its shark fins harvested from the world’s oceans.
The ongoing slashing of shark fins from live sharks (only for the sharks to be tossed back into the water and left to die) is not the only controversial aspect of shark fin collection. What one eats in that $100 bowl could be shark fin that has come from threatened or endangered sharks. Scientists from Stony Brook University and the Field Museum in Chicago, with support from the Pew Environment Group, have used DNA barcoding to identify the species of sharks served in shark fin soups sold in major U.S. cities. According to their studies, several at-risk shark species have been identified in these soups, including the scalloped hammerhead, which is listed as endangered by the International Union for Conservation of Nature (IUCN).
DNA barcoding is extremely useful in the identification of shark species used in soups because the processing of fins makes it difficult to distinguish species through traditional taxonomic classification. By the time shark fins are in broth, they have been dried, chemically treated and cut into pieces. After the fins are cooked, there is just enough DNA extracted to generate a DNA barcode, a DNA sequence unique to each individual species.
The continual harvesting of shark fins endangers the livelihood of shark species that are pertinent to the ocean’s ecosystems. And sharks are particularly prone to over-exploitation as they are slowly reproducing creatures. Sharks must be at least in their teens or twenties to be able to reproduce and they can only a give birth to a few pups in their lifetime.
Despite the scientific facts, consistent laws and regulations need to be enforced in order to prompt effective change. According to the U.S. it is legal to use all the shark species found in these soups because none of these species are on the United States Endangered Species List. Neither are they protected by the Convention on International Trade in Endangered Species (CITES). And although it is illegal for fishers in the U.S. to cut fins off live sharks and then toss them back into sea, it is legal for the fishers to import fins from countries that may have less restrictions.
So where does that leave us? What can we do to help fix this? Scientists can help by carrying out more studies that expose shark exploitation and more fervently demonstrate the importance of shark species in our ecosystems. Some of us in politics can help generate uniform and more stringent policies that help protect shark species. For the rest of us, we have a significant opportunity to increase the awareness of this problem by educating our friends and family. The last and certainly not least thing we can do is something quite simple. We can refuse to eat in restaurants that serve this soup and certainly we can refuse to eat the soup itself. Plus, with that money, we can each eat at least 6 yummy bowls of ramen.
For more information please go to:
Your Pricey Shark Fin Soup May Also Include Endangered Species
New DNA Study Reveals Fins of Endangered Shark in U.S. Soups
In the Soup, a Dash of Biodiversity
Can a moisturizer treat cancer? 17 Aug 2012 1:30 PM (12 years ago)
How often do you moisturize your skin? Every day? Once a month? Well researchers at Northwestern University in Chicago have given a moisturizer the ability to perform RNA interference and regulate genes.
Topical treatments are common for skin cancers like melanoma, as they can be applied directly to the affected cells. But our skin is very effective at blocking toxins getting into our bodies so the challenge was how to cross that barrier.
Again, enter the realm of nanotechnology, a topic I post about regularly.
This time, the scientists paired gold nanoparticles with small interfering RNA (siRNA) molecules to form a siRNA “sphere.” These miniscule balls were able to penetrate skin cells, and then the specifically-designed siRNA was able to effectively switch off the EGFR gene that codes for the epidermal growth factor receptor protein. EGFR is one of the crucial proteins in pathways to cancer, and can cause cancer cells to go into overdrive and proliferate.
The key factor was the sphere shape, concentrating the nucleic acid in the RNA. Linear nucleic acids can’t get into cells, but spherical ones can.
So what miraculous moisturizer did they use? La Mer? Clinique? Something mixed up in a special laboratory? Nope. They used a cheap, readily available moisturizer.
This type of breakthrough is yet another example of the brilliant strides science can make when one discipline talks to another. In this case, dermatology, cancer and chemistry came together under the remit of the Skin Disease Cancer Research Center at the Feinberg School of Medicine at Northwestern.



