All eyes on Japan: Allogeneic iPSC therapy for limbic stem cell deficiency 16 Apr 4:00 AM (2 days ago)
A brief recap
You might recall Johanne Provost’s story, which I covered in the September edition of RMNU🔬. New details have since emerged regarding the specifics of her disease: Ms. Provost had been suffering from a rare autoimmune disease – lichen planus – that destroyed the limbal stem cells maintaining the transparent layer of tissue over each corneal surface.
As mentioned in my previous highlight, the destruction resulted in an aberrant fibrosis obscuring her vision and causing her significant pain. To address this deficiency, her health care team at Toronto’s University Health Network (UHN) ultimately decided she was a suitable candidate for a limbal stem cell transplant from a deceased donor. The usual route would have been to graft healthy stem cells from her other eye but, unfortunately, both corneal limbi were affected.
Though this procedure successfully restored Ms. Provost’s sight, she will have to remain on immunosuppressive medications. The cornea surgeon on her UHN team, Dr. Clara Chan, commented on this for The Globe and Mail:
“Not every patient is able to tolerate these strong drugs and even if they can, there are many patients who remain at high risk for rejection. […] My hope is that there will be ways that we can modify the limbal stem cells to avoid triggering the recipient’s immune system, which would lessen the medication regimen for these patients.”
The latest on a world-first
Research published in The Lancet late last year (November 2024) appears to have brought us closer to realizing this goal, though there’s still some distance to go. There’s been plenty of media coverage on this story, but I believe there are important details that require expanded discussion.
Dr. Kohji Nishida’s team at Osaka University published the results of a small trial to ascertain the safety of an induced pluripotent stem cell (iPSC)-based treatment for limbal stem cell deficiencies. Generally, this type of deficiency can be caused by conditions including autoimmune disorders, thermal and chemical burns, or Stevens-Johnson syndrome.
A critical factor in the Osaka study, however, was that the iPSCs were not generated from the patients themselves. The intention was to develop an allogeneic therapy, where donor blood cells were reprogrammed and differentiated into a sheet of corneal epithelium that would ultimately replace the patients’ scarred ocular tissue. The reason why – along with the trade-off – matters greatly in this context.
Pros and cons
A key advantage of autologous rather than allogeneic iPSC therapies is that they have the potential to eliminate the need for immunosuppressive medications. Since the treatment uses the patient’s own cells, the risk of immune rejection is significantly reduced. However, similarly to other groups discussing “off-the-shelf” iPSC therapies, the authors argue that an approach involving patient-derived iPSCs also poses disadvantages, including matters of cost and time efficiency.
Despite being more immunogenic, allogeneic stem cells are proposed to be more readily available if produced ahead of time in bulk, which could additionally reduce overall treatment costs. Of course, an off-the-shelf approach would not be without its own set of logistical and quality-assurance challenges, including manufacturing consistency, genomic instability, storage, and the potential for disease transmission. Go to Madrid et al.’s November 2024 white paper if you’re interested in more information on this topic.
While there are efforts ongoing to reduce or eliminate the immunogenic properties of off-the-shelf iPSC therapies, the authors further argue that their specific disease case offers unique advantages in this regard. Previous experiments have shown lab-grown corneal epithelial cell sheets to have limited immunogenicity as assayed in cynomolgus monkeys. The lowered threat profile of this tissue comes down to two features:
1) The cells’ immune-related gene expression profile is advantageous, as HLA I and II are present at much lower levels. HLA proteins are cell surface molecules that present antigens to the immune system, and downregulation of HLA I and II would reduce the likelihood of the recipient’s immune system recognizing and rejecting transplanted tissue.
2) Lab-grown corneal epithelial cell sheets have an ideal cellular composition. The tissue does not contain immunocompetent cells and is fabricated exclusively from induced corneal epithelial progenitors, unlike certain cadaver-sourced materials.
Originally, these qualities led the authors to hypothesize that the use of immunosuppressive agents beyond corticosteroids would not be necessary for their treatment (although preclinically, mild immunosuppression was deployed). However, they still tested this requirement in their experiments, and it’s a good thing they did. The trial was designed such that two of the four patients were on corticosteroids, and the other two were administered corticosteroids plus low-dose cyclosporine.
Two years post-treatment, none of the four patients presented with severe side effects such as tumour formation. Critically, while the immune response was not sufficient for the tissue to be rejected in the classical sense, patients did not appear to be completely immunotolerant of the cells; indeed, the immunosuppressed group exhibited more pronounced visual improvements, suggesting that subclinical immune rejection may have been a factor in the corticosteroid-only group.
The bottom line
It is important to bear in mind that we only have data on four subjects. Given the (understandably) limited nature of the study, a much larger trial is being organized to determine efficacy. This was slated for March 2025, though there is no news yet. For now, the data do afford a preview of the treatment’s potential: All four patients experienced immediate vision improvements. It should be mentioned that these effects were durable in only three subjects; one individual’s particularly severe underlying condition is thought to have caused their regression to baseline.
Once again, the ultimate goal will be to leave immunosuppressive regimes behind completely, an important element that will require protocol optimization. If the next trial is successful, it may pave the way for autologous or engineered, virtually non-immunogenic allogeneic iPSC treatments in future. For now, the Osaka strategy may still mark an improvement as compared to the current benchmark, since rejection rates are quite high (40 per cent, according to the authors) following standard corneal limbal transplants, such as those from cadavers.
I’ll continue to monitor the latest on this line of investigation in upcoming editions of Regenerative Medicine News Under the Microscope, so make sure to check back for updates.
Right Turn: Ethics in science communication 8 Apr 4:00 AM (10 days ago)
 As a communication professional, I know firsthand that communication plays a vital role in shaping relationships between organizations and stakeholders. By upholding ethical standards, such as the International Association of Business Communicators’ Code of Ethics for Professional Communicators and the Canadian Public Relations Society’s Code of Professional Standards, communication professionals like myself work to ensure transparency, trustworthiness and integrity on behalf of our employers.
As a communication professional, I know firsthand that communication plays a vital role in shaping relationships between organizations and stakeholders. By upholding ethical standards, such as the International Association of Business Communicators’ Code of Ethics for Professional Communicators and the Canadian Public Relations Society’s Code of Professional Standards, communication professionals like myself work to ensure transparency, trustworthiness and integrity on behalf of our employers.
With ethical guidelines top-of-mind in my daily work, I started to wonder whether a comparable set of standards exists for scientists, researchers and companies when practicing science communication. Science communication refers to the sharing of scientific research and its potential applications with the public (for background information on the topic, check out a treasure trove of other science communication-related posts on Signals here).
Underlying my thinking is that since groundbreaking technologies in the stem cell and regenerative medicine field hold the promise of transforming health care, abiding by a set of ethical standards when sharing advances is critical for ensuring the public gains an understanding of where the science is at today, and its potential for the future.
In addition, a recent episode of CCRM’s podcast, Commercializing Living Therapies with CCRM, called “Unapproved stem cell therapies: Risks, realities and responsibilities,” highlights the downsides of what I would consider unethical science communication. The episode covers the consequences of clinics that sell unapproved treatments targeting desperate or uninformed patients with ads, social media posts, etc., to drive demand for potentially risky procedures.
As I conducted research for this post, I learned that a formal set of ethical standards for the global science communication community does not exist (yet?). That said, institutions, organizations or professional associations may have their own ethics policies that may apply to science communication and/or professional behaviour generally. For example, I found that the U.S.-based National Association of Science Writers has a code of ethics and conduct for its members, the Government of Canada’s National Research Council has a policy on research and scientific integrity, and Engineers Canada has its own code of ethics.
Fortunately, the Journal of Science Communication published a 2017 article suggesting that the Mertonian norms, a set of four principles that describe the values and practices of modern science, can guide science communicators, whether or not they are governed by a separate policy. These principles are noted below, accompanied by examples illustrating each one in action.
Principle 1: Communalism (Common ownership of scientific knowledge)
An example of how a science communicator can integrate this principle is ensuring that publicly funded research is shared openly with broad audiences, avoiding paywalled journals as the sole sources of information (as a first step, reference the University of Oxford’s tool for finding open-access publications here). Accessible language is used to summarize findings. The goal is to avoid the privatization of knowledge, and society can benefit from understanding (and accessing) scientific discoveries.
Principle 2: Universalism (Science should be evaluated independently of who conducts it)
When covering new research, a science communicator should focus on the validity of the study’s methodology and findings, rather than the prestige of the institution or the researcher’s professional background. This would reduce bias and promote trust in science based on evidence rather than authority or reputation.
Principle 3: Disinterestedness (Scientists should act for the benefit of knowledge, not personal gain)
A science communicator must disclose potential conflicts of interest when discussing scientific breakthroughs, such as funding sources or industry ties. They should avoid sensationalism and exaggerating results for personal or organizational gain. This can help ensure audiences receive objective and trustworthy information rather than narratives that serve particular interests.
Principle 4: Organized Skepticism (All scientific claims should be critically scrutinized)
The science communicator should present findings with appropriate caveats, emphasizing that science is an evolving process. It’s essential to fact-check claims, cite peer-reviewed sources, and encourage audiences to think critically rather than accept information at face value. By fostering a culture of healthy skepticism, we can prevent the spread of misinformation and encourage evidence-based decisions.
By prioritizing the four principles outlined above, and considering the long-term societal impact, we can navigate the intersection of science communications and ethics effectively, fostering trust and accountability.
For more on ethics in science communication, watch the recording of a robust panel discussion hosted by the U.S.-based Institute for Science & Policy in 2023, called “Ethical Boundaries and Obligations of Communicating Science,” in the video below.
The first pancreatic organoid with all pancreatic cell types 3 Apr 4:00 AM (15 days ago)

Image of a section of the pancreatic organoid, visible through a microscope. The cell nucleus is in blue, somatostatin is in green and C-peptide is in magenta. Source: Dr. Amanda Andersson-Rolf.
The pancreas is an organ that is critical for the digestion of our food and for releasing hormones into the bloodstream, which helps regulate blood sugar levels. The three main types of cells responsible for making these functions happen are: endocrine cells, acinar cells and duct cells.
You most likely have heard of beta cells due to their role in diabetes. Beta cells are endocrine cells that release insulin, which signals cells to intake glucose from the blood. Alpha cells, which I like to think of as the opposite of beta cells, release glucagon, signalling cells to release glucose into the blood. Both are in a delicate balance. Other pancreatic endocrine cells, such as somatostatin and ghrelin, regulate glucose homeostasis and satiety signalling.
Unlike endocrine cells, which release hormones directly into the bloodstream, acinar cells release their enzymes into a tubular network of ductal cells. They are transported through ductal tubules and land in the small intestine to digest our food. The digestive enzymes, such as amylase, lipase and trypsin, help break down sugars, fats and peptides, respectively.
Organoids are 3D miniature organs that grow from stem cells that can produce all or some of the different kinds of organ-specific cells as part of their progeny. Therefore, some organoids reflect the diversity of cell types found in a single organ, as well as the interconnective network between the various cell types. Observing how different cell types interact with each other and the environment helps scientists understand what makes a healthy functional organ.
If organoids are created by a human stem cell (rather than a mouse stem cell) it offers a way to model 3D human organs. Organoids have become a popular model system in the scientific community because these self-organizing 3D structures can mimic human organ generation and they have the potential to overcome the limitations of using animal models for drug discovery (for example, high failure rates in clinical trials and ethical concerns). Intestinal organoids and brain organoids have been successfully cultured and grown for a while now. However, there is something about the pancreas organ that has made it extremely difficult to model in its entirety in the lab.
Previously, scientists have only been able to create pancreatic organoids made exclusively of endocrine cells or organoids made entirely of duct cells. Since these organoids do not contain all three pancreatic cell types (or the progenitors that make all three of them), they do not reflect the cellular heterogeneity found in the human pancreas organ.
Creating a pancreatic organoid with all three cell types has not been possible until now. Dr. Amanda Andersson-Rolf, who was a post-doctoral fellow in the Hans Clevers lab (at Hubrecht Institute, Netherlands) and her team, have finally created an organoid that has all three differentiated cell types. To establish their organoid, the group took cells directly from the human fetal pancreas (from the first and second trimester).
Depending on what media you give the organoid, it will produce more endocrine cells or more acinar cells.
These endocrine cells and acinar cells can produce their corresponding hormones and digestive enzymes, which is great, but can these cells also release them? The answer is yes! The researchers were successful in showing that beta cells release C-peptide (this shows that they most likely also release insulin) and that acinar cells can release their corresponding enzymes.
This fact is important because being able to actually release the hormones and digestive enzymes is indicative of a more mature, differentiated functional cell. In the long term, the ability to generate endocrine and acinar cells provides scientists with the possibility to study Type 1 diabetes (which affects endocrine beta cells) as well as pancreatic acinar cell carcinoma (which affects acinar cells). The ultimate goal is to be able to generate these cell types for regenerative therapies and identify drug targets for pancreatic cancers. Although far off into the future, scientists would also like to someday use the whole organoid as a tool for organ transplantation due to organ donor shortages.
For the pancreas, we may be a long way off from being able to transplant the whole organ, especially since even the mature cells within the organoid created by the Hans Clevers lab still resemble the first/second-trimester fetal stage rather than adult cells. However, being able to create the pancreas organ with all its cell types inside a dish means we can study how all these cell types interact with each other, develop, and ultimately create the human pancreas. It could also help us understand which types of cells to use to grow a pancreas organoid.
Furthermore, it appears that these organoids, made of tens of thousands of cells, originate from a single stem cell and that this stem cell has a stem cell marker called LGR5 (a type of receptor on the cell surface). Since this stem cell can ultimately differentiate into all three cell types of the pancreas – endocrine, acinar, and duct – we call it tripotent. Knowing that LGR5 labels the human embryonic pancreatic stem cell will make it easier to repeatedly isolate not only the pancreatic stem cell, but its downstream beta cell or acinar cell, which may make it easier to conduct future clinically relevant repeatable studies.
It is interesting to note that for mice, LGR5 expression does not seem to be a pancreatic stem cell or progenitor marker during development or in adulthood under normal conditions (although when the adult mouse pancreas is injured, ductal cells will express LGR5 and act as stem/progenitor cells).
There are many other differences between a mouse pancreas and the human pancreas on a cellular and developmental level. “As a result, it is not always possible to extrapolate from mouse to human. The processes of pancreatic development are slightly different. Timeline-wise, […] we wouldn’t have expected to find tripotent stem cells so late in human development as we do [based on previous studies in mice],” Dr. Andersson-Rolf explained in an interview with me.
That is why one of the main advantages of Dr. Andersson-Rolf’s and her team’s pancreatic organoid is that we can study human pancreas development and how a single human LGR5+ stem cell creates all three differentiated cell types. Although mouse models have been extremely useful, there are inherent species-specific differences between the mouse and human pancreas. For example, organ morphology, the proportion of the endocrine cells, and certain events in development.
Having an organoid that reflects the diversity of human pancreatic cells reduces our reliance on animal models or using pure populations of one homogenous pancreatic cell type (which have little resemblance to human organs) to study disease, developmental processes, or drug therapies that could potentially be used to promote human beta cell regeneration for Type 1 diabetes or reverse human pancreatic cancer.
Specifically, understanding how a human pancreatic stem cell decides to become a beta cell, duct cell, or acinar cell through human organoids will provide insight into how diseases related to the human pancreas develop. Further, organoids derived from human cells that reflect the architecture of the human pancreas would be one of the best ways for scientists to predict how humans will respond to new therapies and to test for the safety and efficacy of pharmaceutical drugs before they reach human clinical trials.
Dr. Andersson-Rolf is now transitioning to becoming a Principal Investigator of her own lab and will continue to investigate pancreas development and disease.
Regenerative Medicine News Under the Microscope – February Edition 26 Mar 4:00 AM (23 days ago)
Some big trends to discuss this month, as well as one-off headlines. I have clinical updates on epilepsy, tooth regeneration and Parkinson’s – a disease area quickly becoming an RMNU🔬 staple, given all the movement in this field! Read on, bookmark for later, or send to a colleague.
Pick of the Month
Phase III trial for epilepsy cell therapy
About one-third of adults with epilepsy, and up to a quarter of afflicted children, have difficulty controlling their epilepsy with medications. With limited treatment options remaining, some patients turn to resective brain surgeries that – while ameliorating seizures – can result in other serious functional impairments.
Neurona Therapeutics is developing an allogeneic, or “off-the-shelf” cell therapy for drug-resistant seizures (NRTX-1001) by bringing balance back to the brain. It’s been shown that GABA neurons – an inhibitory cell type – can counteract the excessive activity or excitation that occurs during a seizure. The aim is to leverage this power by delivering lab-created GABA neurons to disease-linked neuron populations.
NRTX-1001 is manufactured by differentiating human embryonic stem cells to GABA neurons. The cells are then injected into the temporal lobes of patients with seizures stemming from this region. Their Phase I/II trials looked at unilateral and bilateral cases, with work still ongoing. The positive results presented so far with respect to safety (no adverse events attributed to the therapy), but also preliminary efficacy, are spurring the jump to Phase III: In the low-dose group of their unilateral study (n=5), there was a 92 per cent median decrease in disabling seizures relative to baseline measurements 7-12 months post-injection. For their higher dose group (also n=5), 7-12-month data are not yet available, but the researchers are reporting a 78 per cent median drop for the 4-6-month period post-injection.
As a neuroscientist with a deep appreciation for the complexity of brain circuits, I just want to take a moment to emphasize how interesting cell therapies for neurological diseases truly are. The fact that you can send new cells into the brain – relief workers without any instructions or knowledge of the situation on the ground – and they’re able to work with the locals and interpret environmental cues well enough to not only integrate, but also create these early improvements we’re seeing… that’s really something.
The drug was given an RMAT designation in 2024, which helped the team reach Phase III sooner. While early results are promising, larger patient cohorts will be necessary to determine the therapy’s true efficacy. Still, this appears to be the most advanced drug of this nature for epilepsy, so it’s one to watch!
Growing new teeth in vivo
I’ve wanted to write a proper highlight about this topic for a while now, given all the important work being done in the field!
- In brief: Katsu Takahashi’s progress.
In my post-holiday edition released last month, many big headlines needed coverage as main features. That being said, one of my additional recommendations from December was Takahashi’s new Phase I clinical trial examining a simple but promising approach: monoclonal antibodies against USAG-1 in healthy adults missing a tooth. Preclinical studies featured compelling pictures of results in mice and ferrets with fully-formed replacement teeth, so there appears to be a lot of optimism around this investigation at the moment. While the clinical trial is focusing on healthy baseline subjects, the medicine will ultimately be geared towards individuals with congenitally missing teeth.
Curious for more? Read this earlier blog post by Peace Chukwu.
- The bioengineering approach to tooth regeneration.
Now, for the February headline: A team of researchers at Tufts University is also making progress on this front and has managed to grow tooth-like structures in adult minipigs. Their approach? Bioengineered tooth buds. They make them by seeding dental stem cells onto a scaffold made from decellularized tooth bud ECM, with materials sourced from pig jawbones and extracted human teeth. These engineered tooth buds are then implanted into extraction sockets where a minipig’s lost tooth used to be. It’s a completely different approach to Takahashi’s team but, as you can see, researchers are attacking toothlessness from all sides.
Zhang and Yelick did ultimately observe the formation of mature tooth tissue, and while they weren’t perfect in shape or size, the end product did ultimately bear resemblance to the true, all-coveted replacement. They’re not quite ready for human studies according to this NPR Short Wave, but the more options we have down the line, the better. That’s especially true depending on which indication related to tooth loss each treatment is being applied for.
More work to regenerate the Parkinsonian brain
Last month, I updated you on BlueRock’s jump into phase III trials. This month, two more headlines from competitors have crossed my desk:
- AskBio’s RMAT designation for their Parkinson’s disease (PD) gene therapy
Positive Phase Ib data at 36 months led to the FDA’s decision on this new investigational medicine. Now called AB-1005, the drug was formerly referred to as AAV2-GDNF – and yes, the old name is far more literal! It’s an AAV2 packaged with a transgene for human glial cell-derived neurotrophic factor (GDNF) targeted to the putamen, similar to PD stem cell therapies. In preclinical studies, GDNF has been found to enhance the survival and differentiation of dopaminergic neurons, so the aim of this intervention is thus to slow disease progression and ameliorate motor function in PD patients.
I’ll also mention that AskBio is an owned and independently-operated subsidiary of Bayer AG.
Thanks to a lack of adverse events and positive trends reported for Phase I outcomes, enrollment is now ongoing for a Phase II study, called REGENERATE-PD.
- A new autologous iPSC-derived dopamine cell replacement trial
Preclinical results laid out in this February paper by Jeon et al. have set the stage for a new Phase I clinical trial that’s currently recruiting. Fibroblasts were collected from human patients with PD, reprogrammed into induced pluripotent stem cells (iPSCs) and then differentiated into dopaminergic progenitors. These progenitors are then delivered to rodent models of the disease. The clinical trial will be highly similar in format, except that the final recipients of the reprogrammed cells will be eight human PD patients. While a commonly cited advantage of iPSCs is that they can be used with minimal fear of immune rejection, I think it’s an open question as to whether sourcing the cells from a healthy donor might yield stronger or more long-lasting outcomes – especially if genetic elements are in play for a specific patient’s disease etiology. I found some validation regarding this concern in a recent review on the matter. Ultimately, time and trials will tell which is the better approach for whom, especially as scientists try to avoid immunosuppressants and keep infection or cancer risks low. There are pros and cons to any cell therapy approach, and we’re still building those arguments – especially with respect to efficacy data.
If the iPSC-based approach to PD cell therapy sounds familiar to you, perhaps you’re recalling Aspen Neuroscience’s ongoing trial, which I covered back in the May 2024 edition of RMNU🔬. These two groups appear to be testing highly similar concepts, so keep an eye on both – and as always, I’ll update you as the data start to flow.
Additional recommendations
Risdiplam for Prenatal Therapy of Spinal Muscular Atrophy. A rare genetic disorder, spinal muscular atrophy has been treated in utero for the first time. So far, all signs point to success as the baby girl in question appears healthy at two and a half years old. Note that the child will likely have to be on medication for the rest of her life, and longer-term monitoring will be necessary. It’s also an n of one. Still, a highly compelling case indeed.
Individual and additive effects of vitamin D, omega-3 and exercise on DNA methylation clocks of biological aging in older adults from the DO-HEALTH trial. These two supplements are widely discussed for an array of reasons and indications. This one isn’t perfectly in line with the regenerative medicine theme, but I’ll permit it because of how closely related longevity science tends to be. In addition, a lot of us already take these supplements for other reasons, whether or not these findings hold or shake out to be solid. I am not endorsing any supplements here, nor am I a medical doctor positioned to suggest them to others, I only highlight the science. Happy to encourage more exercise though, as I don’t think I need a medical degree for that one. Further reading and analysis can be found at Nature News: Omega-3 supplements slow biological ageing.
Nature-inspired platform nanotechnology for RNA delivery to myeloid cells and their bone marrow progenitors. Many companies and research groups are zeroed in on RNA delivery right now – I’m seeing it come up quite a bit.
New stem cell transplant approach offers potential sickle cell cure.
Bacteria, stem cells and cancer.
Maternal gut microbiota influence stem cell function in offspring.
Retina Stem Cell Treatment to Come Under National Health Insurance.
Experimental cell therapy trial treats first Sjögren’s disease patient.
Stem cell therapy found to reduce itch, pain in RDEB children in trial.
Decoding functional hematopoietic progenitor cells in the adult human lung.
Bioprinting of bespoke islet-specific niches to promote maturation of stem cell-derived islets.
Mesoblast prices pediatric stem cell treatment at $1.55 million.
Astrocyte heterogeneity reveals region-specific astrogenesis in the white matter.
Single-cell atlas of human pancreatic islet and acinar endothelial cells in health and diabetes.
Identification of Meibomian gland stem cell populations and mechanisms of aging.
Neural stem cell relay from B1 to B2 cells in the adult mouse ventricular-subventricular zone.
Unraveling the impact of human cerebrospinal fluid on human neural stem cell fate.
Circulating miRNAs are associated with successful bone regeneration.
Cloned airway basal progenitor cells to repair fibrotic lung through re-epithelialization.
Human fetal lung mesenchymal stem cells ameliorate lung injury in an animal model.
BMP signaling promotes zebrafish heart regeneration via alleviation of replication stress.
AI-based approach to dissect the variability of mouse stem cell-derived embryo models.
Addressing Widening Health Disparities With Inclusive Stem Cell Models.
Other notable FDA approvals, RMATS and status changes
Britecyte’s Adipose Tissue Allograft Earns “Safe to Proceed” Status from FDA.
Bionic Sight’s BS01 Gene Therapy Receives RMAT Designation from the FDA.
Regulatory affairs careers: What scientists need to know 18 Mar 4:00 AM (last month)
 For life science graduates exploring career options, the regulatory affairs function is where scientific expertise meets real-world impact. Regulatory affairs professionals play a crucial role in bringing regenerative medicine – including cell and gene therapies – medical devices and pharmaceuticals to market while ensuring safety and compliance with regulations made by health authorities, such as Health Canada, the U.S. Food and Drug Administration, and the European Medicines Agency.
For life science graduates exploring career options, the regulatory affairs function is where scientific expertise meets real-world impact. Regulatory affairs professionals play a crucial role in bringing regenerative medicine – including cell and gene therapies – medical devices and pharmaceuticals to market while ensuring safety and compliance with regulations made by health authorities, such as Health Canada, the U.S. Food and Drug Administration, and the European Medicines Agency.
Last month, Careers Beyond Academia featured two speakers working in regulatory affairs positions in the regenerative medicine and pharmaceutical sectors. The speakers shared insights and advice for people with life sciences degrees who are considering a career outside of academia. Careers Beyond Academia, a virtual series, is hosted by the University of Toronto’s Medicine by Design and the Stem Cell Network.
The session kicked off with introductions of the two speakers. They were:
- Zoe Anderson-Jenkins, Associate Director – Regulatory CMC, BlueRock Therapeutics
- Tracy Porter, Associate Director, Regulatory Strategy, Vertex Pharmaceuticals
In the session, Zoe and Tracy discussed the skills required for working in regulatory affairs and some of the daily functions of their roles. They then divulged helpful tips for scientists considering entering the field. I’ve included some of their key suggestions below.
Top takeaways for aspiring regulatory affairs professionals
Expand your writing skills. Regulatory affairs professionals write many different types of documents that are required by health authorities. However, scientists working in a lab might not yet have experience with the type of writing that’s required in regulatory documents. Prepare for the transition to a regulatory affairs role by expanding your writing skills and technical writing experience, whether it’s for a blog like Signals (thanks for the shoutout Zoe!), or in manuscripts or grants (these Penn University slides are a good resource).
Build and engage your network. Audit your existing LinkedIn connections to see who is working in regulatory affairs. Also, cast a wider net on LinkedIn to find other professionals with regulatory affairs job titles or experience, and determine who in your network could introduce you to them by identifying common connections or organizations. Ask these people to join you for virtual or in-person informational interviews, during which you can learn about their careers (find some tips to guide your interviews here). While this tactic might seem unappealing if it’s a step outside of your comfort zone, Tracy reiterated that people are generally open to informational interviews because they enjoy talking about themselves and helping others, so rest assured that the risk of rejection is low.
Attend industry conferences. Industry conferences and meetings can provide inside information and an opportunity to network with other professionals. Tracy recommended looking for conferences, meetings or other events organized by the Canadian Association of Professionals in Regulatory Affairs, the global Drug Information Association, and the U.S.-based Regulatory Affairs Professionals Society.
Increase your knowledge. If your company or lab has interactions with health authorities, Zoe recommended volunteering to gain exposure to the types of documents that are being prepared for regulators. Even if you’re only able to read previously submitted documents, this is a great way to familiarize yourself with regulatory content and the level of writing that is fit to submit. Further, Tracy suggested doing research to increase your knowledge of the Canadian regulatory and health care systems (and/or other countries/regions, as applicable) and becoming familiar with the processes and steps required to commercialize a product. Some great places to start are:
- Aileen J. Zhou’s 2022 Signals post on the Canadian regulatory system for cell and gene therapies.
- The episode of the Commercializing Living Therapies with CCRM podcast titled “Regulators’ strategies for managing the surge in cell and gene therapies” from February 2024.
- Laya Kiani’s September 2024 Signals post about navigating regulatory challenges and advancements in the regenerative medicine market.
I’ll end the post with a final thought that Tracy shared, which readers should reflect on before diving into a regulatory affairs career path. Someone once told her: “If you enjoy filling out forms and doing taxes, regulatory affairs might be for you!”
Watch the recorded session below to learn more.
https://www.youtube.com/watch?v=IgqIwcnAqKk&list=PLeXYwnb92ytTY5Ev94__y8M9t3mPqkmeo&index=16
Headwinds and tailwinds for cell and gene therapy under the second Trump administration 11 Mar 4:00 AM (last month)
“Every administration has its headwinds and tailwinds” said Tim Hunt, CEO, Alliance for Regenerative Medicine (ARM), when examining the impact of the two most recent U.S. administrations at ARM’s 2025 State of the Industry Briefing in January.
Credit: Alliance for Regenerative Medicine
Under Trump’s first administration, headwinds mentioned by Mr. Hunt included the immigration ban that biotech executives warned could make it harder to attract talent from overseas, along with efforts to tie Medicare drug prices to foreign benchmarks via the Most Favored Nation (MFN) rule, which attempted to lower U.S. drug prices by tying Medicare reimbursements to the lowest price paid by a selection of foreign countries, including Canada.
Canada controls drug prices through the Patented Medicine Prices Review Board, which sets price ceilings based on an international reference group. Drug developers often set higher prices in the U.S. to recoup research and development costs, which are less subsidized there than in many other countries. Following industry pushback, which argued that the MFN rule discouraged investment in new therapies, along with legal challenges, the rule was rescinded by the Biden administration in 2021 before it ever took full effect.
One enduring tailwind from Trump’s first administration was the appointment of Scott Gottlieb as U.S. Food and Drug Administration (FDA) commissioner, who, Mr. Hunt said, “ended up being a terrific FDA commissioner, certainly for cell and gene therapy from 2017-2019…there was a proposed rule that they did a fantastic job on, that eventually got finalized in the Biden administration, that would really kind of enable outcomes-based arrangements in both the commercial setting and in Medicaid by working through things like Medicaid best price – something the industry really lobbied for and we were delighted when this rule came out.”
Under Trump’s second administration, these efforts look set to continue. “Half of the gene therapy/gene editing patient populations are probably in Medicaid, something around that order,” estimated Mr. Hunt. “It depends on the disease, could be more, sickle cell is higher, with some it’s going to be less, but thinking about ‘how do we modernize Medicaid to embrace cell and gene therapy’ is a major advance for our field, so a major tailwind there.”
Mr. Hunt sees real opportunities in the alignment between what the industry promises to deliver and some of the stated goals of Trump’s second administration: particularly in terms of the desire to address the root causes of diseases and “get away from chronic care, the pills of the past, on some level,” as Mr. Hunt put it.
Credit: Alliance for Regenerative Medicine
One headwind on many minds is the U.S. National Institutes of Health’s (NIH) attempt to cut indirect funding, capping the overhead payments that institutions receive to support research infrastructure at 15 per cent. The American Society of Gene and Cell Therapy released a statement expressing concern, joining dozens of leading research universities that have released statements and joined forces to challenge the cuts in federal court (separate from the lawsuit filed by the attorney generals of 22 different U.S. states). As of this writing, the cuts remain blocked by a nationwide injunction issued March 5, with the Trump administration expected to appeal.
Some have gone as far as to describe the attempted cuts as a “Science Apocalypse,” so it is only fair to include the rationale that NIH gave when announcing them, stating “Last year, $9B of the $35B that the National Institutes of Health (NIH) granted for research was used for administrative overhead, what is known as “indirect costs.” Today, NIH lowered the maximum indirect cost rate research institutions can charge the government to 15%, above what many major foundations allow and much lower than the 60%+ that some institutions charge the government today. This change will save more than $4B a year effective immediately.”
Back over to tailwinds to finish on a positive note: Ongoing regulatory innovation spearheaded by Dr. Peter Marks, Director, Center for Biologics Evaluation and Research (CBER) at the FDA was mentioned by two speakers on a commercially-focused panel – Capital markets and commercial insights: navigating opportunities and challenges in CGTs – which formed part of the ARM briefing. Each panelist shared thoughts on tailwinds to come:
Dr. Kinnari Patel, President, Head of R&D and Chief Operating Officer, Rocket Pharma:
“Administrations may come and go… but the regulations, the people and teams have been strengthened, so I’m really excited about that… the momentum being built in CBER specifically….” Dr. Patel also cited collaboration with the European Medicines Agency – discussed in this Signals blog post – as a positive sign.
Dr. Jim Birchenough, Chairman and Global Co-Head, Barclays Biopharmaceutical Investment Banking:
“I heard this weekend a lot of talk about AI in different facets of drug development, but at FDA, actually being used to do the mundane work that can free up reviewer time to have direct interactions on a more frequent basis with sponsors, so that’s something that we’re watching very closely, it could be a very positive dynamic.”
Keith Crandell, Co-founder and Managing Director, ARCH Venture Partners:
“I’m sort of expecting a little bit of confusion over the next few months…I think it’s sort of incumbent on the boards of the companies that are involved and also on the management to really reach out and focus on education… I think there is a tremendous amount of momentum behind the industry, and I think most political groups like success, and I think that this set of technologies and curative therapies offers them a tremendous amount of wins.”
Lynelle Hoch, President, Cell Therapy Organization, Bristol Myers Squibb:
“What we are excited about is this Administration seems to have a bold mindset of he [Trump] wants to lead in science, and he believes in innovation and he believes in what it takes to innovate. So we do hope that we can harness that mindset to be able to forge ahead as a leader overall in cell therapy, as a country but also as an industry.”
The impact of IPOs on biotech market expansion: Strategies and projections 6 Mar 4:00 AM (last month)
The biotechnology sector has always been a hub of innovation, consistently pushing the boundaries of scientific and technological advancements. However, transforming novel discoveries into viable market-ready solutions requires substantial financial backing. Among the different ways biotech and pharmaceutical companies secure long-term financial stability, Initial Public Offerings (IPOs) have become a key strategy for raising capital and supporting growth. By transitioning from private to public ownership, these companies gain access to the critical capital needed to increase research capabilities, strengthen investor trust, and fast-track the commercialization of their scientific discoveries.
Over the past two decades, IPOs have played a vital role in facilitating the growth of biotech and pharmaceutical companies. Between 2001 and 2023, nearly 24 per cent of all global IPOs came from these industries, with that number rising to 35 per cent between 2019 and 2023. This steady increase underscores the growing influence of biotech IPOs in the global financial market.
Biotechnology has attracted significant attention from Wall Street, driven by groundbreaking scientific advancements and the successful development of new drugs. The industry thrived in 2021, with more than 100 biotech companies going public and raising nearly US$15 billion. However, this momentum slowed in the following years as broader market downturns led to a decline in IPO activity. Despite these challenges, biotech companies continue to use IPOs as a flexible financial strategy to access global investor networks. Beyond raising capital for research and development (R&D), going public also boosts a company’s credibility and visibility, making it more attractive to investors, partners and key stakeholders in the industry.
The multifaceted benefits of IPOs
IPOs for biotech and pharmaceutical companies serve as more than just a means of raising capital. Beyond their financial benefits, they provide opportunities for companies to improve internal processes, enhance operational efficiency, and prepare for the regulatory demands of being publicly traded. Successful biotech firms that navigate the IPO process effectively tend to share several key attributes, including:
- A commanding market position with a competitive edge in their respective niches
- Clearly defined strategic objectives that align with long-term industry trends
- Robust financial reporting systems to ensure transparency and accountability
- Experienced leadership teams with a deep understanding of the sector’s complexities
- A solid corporate governance framework that supports sustainable growth
IPOs and the need for R&D financing
The biotech sector’s heavy reliance on R&D underscores the critical role of IPOs in financing ongoing innovation. Traditional bank loans are often out of reach for biotech firms due to the high risks associated with long development timelines for drug discovery and approval. As a result, equity financing through IPOs has become an essential strategy for securing capital. This necessity is captured in the phrase “public or perish,” coined by Yuji Honjo and Sadao Nagaoka, to describe how biotech start-ups – particularly those engaged in costly clinical research – are often compelled to go public to sustain their R&D efforts and bring groundbreaking innovations to market.
Beyond just funding individual companies and spurring industry, public investment in R&D plays a crucial role in fostering collaboration and strengthening competitiveness. The impact of IPOs is further amplified by emerging technologies and strategic partnerships, which maximize the value of these investments and ensure long-term sustainability. Additionally, mergers and acquisitions (M&A) serve as complementary financial strategies, helping biotech firms manage risks and accelerate drug development. By integrating IPOs with strategic alliances and M&A transactions, biotech companies can build strong financial ecosystems that support their long-term growth and ability to bring transformative therapies to market.
Investor dynamics in biotech IPOs
Investors play an indispensable role in the success of biotech IPOs, particularly given the industry’s unique challenges, including extended development timelines, significant R&D costs, and stringent regulatory requirements. Pre-commitments and insider participation by investors often serve as stabilizing factors that help maintain offer prices, streamline IPO timelines, and increase the overall likelihood of success. Biotech firms must carefully manage the diverse expectations of venture capitalists, hedge funds and mutual funds to ensure a stable, long-term financing structure.
Insider purchasing during IPOs often signals strong investor confidence and aligns with venture capital strategies, where early-stage, high-risk investments transition into later-stage opportunities with lower risks and faster exit strategies. The timing of these exits is influenced by multiple factors, including stock liquidity, market conditions and post-IPO ownership structures. This underscores the importance of strategic capital management in ensuring long-term investor confidence and financial sustainability.
While IPOs are crucial for biotech companies, they represent only a small fraction of the overall funding required for R&D and commercialization. For instance, IPOs make up just about three per cent of the overall financing in the biotech sector, while strategic alliances and M&A account for a much larger share—around 65 per cent. This disparity highlights the need for a multifaceted approach to financing, where IPOs function as just one component of a broader financial strategy that incorporates various funding sources and investment mechanisms.
Challenges facing biotech IPOs
Biotech IPOs, while strategically important, face a unique set of challenges due to the industry’s inherently high-risk nature. With prolonged development timelines, high failure rates, and limited revenue before regulatory approval, attracting investors can be difficult. Market volatility further complicates the landscape, as economic downturns and shifting investor sentiment can disproportionately impact biotech equity values. To mitigate these risks, companies must carefully time their IPOs, aligning them with favourable market conditions to maximize funding potential while minimizing dilution risks.
A major hurdle in this process is valuation. Unlike traditional industries, biotech firms often lack revenue, making it difficult to apply standard valuation models such as Comparable Company Analysis (CCA), Discounted Cash Flow (DCF), and the Dividend Discount Model (DDM). This often results in mispricing by underwriters, leading to underpricing—which leaves companies with less capital—or overvaluation, which can lead to poor long-term stock performance. Additionally, external factors such as investor sentiment, market demand, and corporate narratives can overshadow fundamental valuation metrics, further complicating the IPO process.
Beyond valuation, capital efficiency is another key challenge. Biotech firms require significant funding to sustain long-term research and clinical trials, yet unlike other industries, they must rely on continuous investor support before reaching commercialization. The unpredictable nature of regulatory approvals adds to the uncertainty, as delays in clinical trials can push back product launches and affect stock performance. Intellectual property protection also plays a vital role, as securing strong patents is critical for maintaining a competitive edge. Given these complexities, biotech firms must engage in strategic financial planning, ensuring efficient capital allocation to sustain operations, reach key development milestones, and maintain investor confidence in the long run.
Looking ahead: IPO landscape in 2025
As we move into 2025, the biopharma and biotech industries are entering a new era of opportunity, driven by declining capital costs, an increasing need for innovation, and evolving policy landscapes. Companies are focusing on replenishing their pipelines through strategic acquisitions, collaborations and targeted partnerships. While smaller, targeted deals will remain prevalent, the potential for larger megadeals cannot be overlooked, particularly as companies seek to bridge growth gaps and diversify their portfolios.
Technological advancements, particularly in artificial intelligence (AI), are also reshaping the biopharma sector. AI applications are evolving beyond drug discovery to enhance clinical trial design, streamline regulatory submissions, and optimize operational efficiency in manufacturing. These advancements are driving strategic partnerships between technology firms and biopharma companies, paving the way for transformative growth in the sector.
Biotech IPOs have proven to be a powerful tool for fueling innovation and expanding the industry, providing companies with the capital, credibility and connections needed to bring groundbreaking therapies to market. However, the road to going public is not without its challenges. From long development timelines and uncertain regulatory approvals to valuation complexities and market volatility, biotech firms must navigate a landscape filled with risks. Despite these hurdles, IPOs remain an essential part of the biotech growth story, offering companies the opportunity to scale their research and accelerate commercialization.
Looking ahead to 2025, the biotech sector is entering an exciting phase, driven by advancements in AI, strategic partnerships and evolving financial strategies. While IPO activity may fluctuate with market conditions, companies that plan carefully, focus on capital efficiency, and build strong investor relationships will be better positioned for long-term success. By blending IPOs with mergers, acquisitions, and collaborations, biotech firms can create a sustainable financial foundation that not only drives profitability but also ensures that life-changing therapies continue to reach the patients who need them most.
Regenerative Medicine News Under the Microscope – Post-Holiday Edition (December 2024, January 2025) 24 Feb 4:00 AM (last month)
I’m back with a post-holiday double edition of RMNU🔬! I cover stem cell patches for failing hearts, a novel skeletal tissue that’s been (re-)discovered, an update on BlueRock’s Parkinson’s therapy, and more. Bookmark, or dive right in!
Pick of the Month(s)
 Stem cell patches for failing hearts
Stem cell patches for failing hearts
Around the globe, around 5000 heart transplants are completed each year. Is that less than you imagined? If so, you’d be justified in expecting more; the supply of hearts simply cannot keep up with the demand created by almost 50,000 additional patients on waiting lists. Given that heart disease is the world’s leading cause of death, this is a major problem.
Last month, Jebran et al. reported a 2021 case study wherein a 46-year-old woman with heart failure received 10 off-the-shelf patches made from stem cells. These were intended as a potentially stabilizing measure, testing whether this treatment could re-muscularize her heart as she waited for a transplant. Fortunately, she received her new organ three months later, allowing the patched one to be examined by the authors.
In the past, cardiac treatments involving stem cells – or stem cell-derived muscle tissues – have resulted in complications including irregular heartbeat, tumour growth, and immune rejection. Such procedures involved either implantation or direct injection of the cell therapies into the organ.
To avoid these serious complications, Jebran et al. designed their patches, also known as epicardial engineered heart muscle allografts, to be stitched onto the outside of the heart. This means they do not fully integrate into the old tissue, though blood vessels were found to infiltrate and feed the therapeutic cells. Instead of integrating, the new cells responded to the heart’s movement, allowing the organ itself to lead the way while enhancing the overall strength of each pump. Metrics obtained from macaque data reported in the same paper included a 15 per cent increase in the heart wall’s thickness relative to controls, plus a 10 per cent uptick in the volume of blood pumped per heartbeat six months post-operatively.
The patches were made by first differentiating induced pluripotent stem cells (iPSCs) into heart muscle and connective tissue. These cells were then incorporated into a collagen gel. Because the grafts were created using materials from an allogeneic source, immunosuppressants were required. However, the authors opted for the type that might’ve already been used for the heart transplant itself (tacrolimus and methylprednisolone).
Another 15 patients have received these patches as part of an ongoing trial. In addition, new patch designs that minimize the need for immunosuppressive drugs are also being tested.
For a longer summary of this story, check out Miryam Naddaf’s piece in Nature News.
Other cardiac-themed research studies getting attention:
- Cardiac bridging integrator 1 gene therapy rescues chronic non-ischemic heart failure in minipigs.
- Follistatin from hiPSC-cardiomyocytes promotes myocyte proliferation in pigs with postinfarction LV remodeling.
New textbook editions incoming: (Mostly) novel skeletal tissue discovered
Thanks to work by Ramos et al. at the University of California Irvine, we now have a new type of tissue on record: Lipocartilage. It can be found in the mammalian ear, nose, larynx and sternum. It’s best suited for flexible body parts, such as the earlobes or the tip of your nose, and is thus more pliable than tougher knee cartilage, for example. Why does the title read, “mostly novel”? Well, lipochondrocytes were first observed by Franz von Leydig – yes, the namesake of the Leydig cell – in 1854. His observation was then somehow lost to time, until now.
Functionally, lipocartilage holds promise as a positive new addition to the reconstructive toolkit. This is especially true for facial defects or elective augmentations, injuries and diseases that result in damaged cartilage: think cleft palates, a damaged larynx caused by cancer, or even rhinoplasties. Current methods in surgeries necessitating cartilage are highly invasive and/or aren’t always the best biomechanical match, including harvesting from a patient’s rib before subsequent transplant to the desired region. Silicone also tends to be used. Fortunately, the future does look brighter on this front: The authors detected lipocartilage not only in vivo, but also in human cartilage cultures grown in vitro using embryonic stem cells. This means they’re already accessible to scientists for regenerative medicine applications, and some teams will now be looking to iPSCs as a potential source.
From function to physiology, these cells are quite unique: lipochondrocytes are stable reservoirs of lipids that do not change size in response to calorie intake or diet. Fat breakdown is prevented in the cells’ vacuoles, keeping their structures consistent. Lipocartilage thus remains soft, pliable and supportive, similar to bubble wrap (according to the authors). That these cells derive their biomechanical properties from the organelles inside of them is a stark contrast to traditional cartilage, which is dependent on extracellular matrix for its sturdiness. Interestingly, even their fat comes from a different source than the well-known adipocyte: Lipochondrocytes produce their own using glucose, while adipocytes absorb dietary fat.
The team looked at rodents and human fetal tissue, but also 65 other mammalian species. Their investigation even extended to bats, where lipocartilage was found to be patterned; parallel ridges enhance the animals’ hearing by attuning them to specific sound waves. However, lipochondrocytes were not found in non-mammalian species, raising questions about why this cell type might be unique to class Mammalia.
For more on this story, check out Aguayo and Selleri’s Science Perspectives piece.
BlueRock’s bemdaneprocel heads to Phase III
You might recall my coverage of BlueRock Therapeutics’ stem cell therapy for Parkinson’s disease (PD), bemdaneprocel, posted last year. They had just received the U.S. Food and Drug Administration’s (FDA) RMAT designation following positive results in a Phase I clinical trial with 12 participants. At the time, they were slated to initiate their Phase II trials; this is no longer the case, as they’ve just been authorized to head into Phase III given the positive data thus far. If the trial goes well, regulatory submissions for marketing authorization will follow, as this jump to Phase III is intended to bring the drug to market sooner (should it perform as expected).
A quick refresher: BlueRock’s protocol involves the reprogramming of embryonic stem cells to dopamine precursor cells, which are subsequently implanted into the patient’s brain. Their therapy was found to be well-tolerated with no major safety concerns over the course of a 24-month follow-up period (with immunosuppressant drugs administered for the first year). While this trial was not necessarily designed to assess efficacy, as Phase I is mainly for safety and dosing, patients did appear to improve with regard to exploratory clinical endpoints – particularly those in the group receiving a higher dose.
Moving into the next phase of study now, exPDite-2 (which does not yet appear to be in the registry) is a double-blind trial designed to assess the efficacy, safety and overall impact of bemdaneprocel. Approximately 102 participants with moderate PD are anticipated.
Notably, this trial will be the first Phase III study involving an allogeneic pluripotent stem cell-based therapy for PD. Read Bayer’s full release here.
See also the following headline from their iPSC-based competitors, Aspen Neuroscience:
First FDA-Approved Mesenchymal Stromal Cell Therapy
This past December, the FDA approved Ryoncil (remestemcel-L-rknd), an allogeneic bone marrow-derived mesenchymal stromal cell (MSC) therapy developed to treat steroid-refractory, acute graft-versus-host disease (GVHD) in pediatric patients.
You may or may not be surprised to hear that the U.S. is not the first country to approve this drug; in fact, Canada granted Prochymal – as it was called back then – conditional approval back in 2012, and in doing so was considered the world’s first regulatory authority to approve a stem cell-based therapy (though again, it was conditional pending further data). New Zealand also conditionally approved the drug that year. Despite these green lights, subsequent clinical use seems to have been limited. Osiris ultimately licensed the intellectual property and sold the drug to Mesoblast for US$50 million in 2013. Mesoblast worked to optimize manufacturing, and rebranded the drug as Ryoncil.
On the other hand, in Japan, the product was licensed to JCR Pharmaceuticals Co. Ltd. and branded as TEMCELL. It was here that the drug’s first full, non-conditional approval was handed down in 2015.
The drug’s approved indication is acute GVHD, which is a serious complication that usually occurs in the first 100 days following an allogeneic stem cell transplant. Donor stem cells develop into the recipient’s new immune system, but aberrantly attack healthy cells as though they’re foreign, causing damage to tissues and organs. Some patients never develop GVHD, but it can occur in up to 40 per cent of cases, ranging from mild to severe. The skin, liver and intestines are often affected, but other organs may be targeted as well. To combat the disease, MSCs produce strong anti-inflammatory effects, modulating the pathological immune response.
For more, check out the FDA’s release linked above, or this summary from the American Association for Cancer Research.
Phase I trial of stem cell transplants for spinal cord injury: Results
The data are officially in from a Phase I clinical trial testing the safety and feasibility of neural stem cell transplantation in the treatment of chronic thoracic spinal cord injuries, which often result in partial or full paralysis. Martin et al. published their results in Cell Reports Medicine just before the holidays last year. Four patients with chronic spinal cord injuries received six bilateral injections of fetal-derived neural stem cells capable of forming neurons and their support lineages, including astrocytes and oligodendrocytes. These particular stem cells were chosen for their preclinical track record of promoting repair without leading to unwanted complications, such as tumour formation. Following surgery, participants were followed for five years.
Two patients demonstrated neurological improvements following treatment, including higher motor and sensory scores, and enhanced electromyography activity in muscles that had previously been considered dormant. Some participants’ pain scores also improved. However, overall mobility and independence scores did not see significant enhancements, emphasizing the need for further research and variable optimization. Of course, being a Phase I trial, only safety and tolerability were meant to be assessed either way, but these early neurological results may hint at therapeutic potential. The researchers are now moving towards a Phase II clinical trial to ascertain the transplant’s true efficacy.
It should be noted that one of the patients in this trial passed away; I didn’t see this in the UCSD release, but it bears mentioning. This was the one serious adverse event recorded in their trial. The participant died of sepsis, likely related to a sacral ulcer 30 months post-transplant. The authors don’t attribute this to the stem cells or the transplant surgery; a reasonable conclusion, given that patients with neurological disorders (and especially spinal cord injuries) have a 25-85 per cent lifetime risk of developing a pressure injury such as that one. However, the authors cannot rule out that the immunosuppression required for the study protocol contributed to the ulcer and infection.
I will keep an eye out for data from Phase II as it rolls in!
Additional recommendations
Modified human mesenchymal stromal/stem cells restore cortical excitability after focal ischemic stroke in rats. So many headlines about this paper – a great addition to your reading list!
Japanese researchers test pioneering drug to regrow teeth. Particularly excited about this one. Imagine a world where losing an adult tooth isn’t a big deal; no more implants, no more bridges – just grow yourself a new one.
Candidate stem cell isolation and transplantation in Hexacorallia. Regenerative medicine… for corals. It still counts.
Black and Asian cancer patients less likely to survive UK stem cell transplant than white peers. This piece in The Guardian covers the following research paper: The impact of patient ethnicity on haematopoietic cell transplantation outcome: a retrospective cohort study on the UK experience
Long-term in vitro expansion of a human fetal pancreas stem cell that generates all three pancreatic cell lineages. See Krystal Jacques’ upcoming coverage of this story for Signals!
Pluripotent stem-cell-derived therapies in clinical trial: A 2025 update.
Synthetic organizer cells guide development via spatial and biochemical instructions.
Adult bi-paternal offspring generated through direct modification of imprinted genes in mammals.
Intermittent fasting triggers interorgan communication to suppress hair follicle regeneration.
Maternal gut microbiota influence stem cell function in offspring.
Self-organization of the hematopoietic vascular niche and emergent innate immunity on a chip.
Refractory myasthenia gravis treated with autologous hematopoietic stem cell transplantation.
Suppression of thrombospondin-1–mediated inflammaging prolongs hematopoietic health span.
Systemic factors associated with antler growth promote complete wound healing.
MRI-guided focused ultrasound for treating Parkinson’s disease with human mesenchymal stem cells.
Investigation of the osteogenic effects of ICA and ICSII on rat bone marrow mesenchymal stem cells.
Advancing ex vivo functional whole-organ prostate gland model for regeneration and drug screening.
OpenAI has created an AI model for longevity science.
Molecular and cellular dynamics of the developing human neocortex.
Bone marrow niches orchestrate stem-cell hierarchy and immune tolerance.
Long-range Atoh1 enhancers maintain competency for hair cell regeneration in the inner ear.
Years after donating a kidney, Alabama woman receives one from a pig.
Extracellular fluid viscosity regulates human mesenchymal stem cell lineage and function.
Baby with spina bifida has promising future after fetal surgery with stem cells.
Stem cell therapy for knees 2025: fact-check, costs, risks.
Updated 2025 List of FDA-Approved Cell and Gene Therapies.
Regenerative properties of bone marrow mesenchymal stem cell derived exosomes in rotator cuff tears.
Exosomes Are Being Hyped as a ‘Silver Bullet’ Therapy. Scientists Say No.
Industry updates from the field of stem cell research and regenerative medicine in December 2024.
Metabolic Reprogramming of Neural Stem Cells by Chiral Nanofiber for Spinal Cord Injury.
Delivery of Prime editing in human stem cells using pseudoviral NanoScribes particles.
This Next Generation IVF Startup Facilitated The Birth Of A Baby For The First Time.
Stroke-induced neuroplasticity in spiny mice in the absence of tissue regeneration.
Myoblast-derived ADAMTS-like 2 promotes skeletal muscle regeneration after injury.
Stem-cell therapy during Kasai effective, safe in biliary atresia: Trial.
Conversion of placental hemogenic endothelial cells to hematopoietic stem and progenitor cells.
Attenuation of skin injury by a MARCO targeting PLGA nanoparticle.
Ageing limits stemness and tumorigenesis by reprogramming iron homeostasis.
An injury-induced mesenchymal-epithelial cell niche coordinates regenerative responses in the lung.
The World’s First Crispr Drug Gets a Slow Start.
Aurion’s regenerative eye disease med improves vision in phase 1/2 trial.
Spatial transcriptomic clocks reveal cell proximity effects in brain ageing.
Oscillatory fluid flow enhanced mineralization of human dental pulp cells.
The nuclear matrix stabilizes primed-specific genes in human pluripotent stem cells.
NAC regulates metabolism and cell fate in intestinal stem cells.
Timely TGFβ signalling inhibition induces notochord.
Mesenchymal Stem Cells-Derived Extracellular Vesicles for Osteoporosis Therapy.
Lentiviral Gene Therapy with CD34+ Hematopoietic Cells for Hemophilia A.
Other notable FDA approvals and RMATS
FDA approves Humacyte’s off-the-shelf artery implant for vascular trauma repair.
FDA approves mini version of RECELL GO for smaller wounds.
Freeze-thawing neural stem cells 19 Feb 4:00 AM (last month)
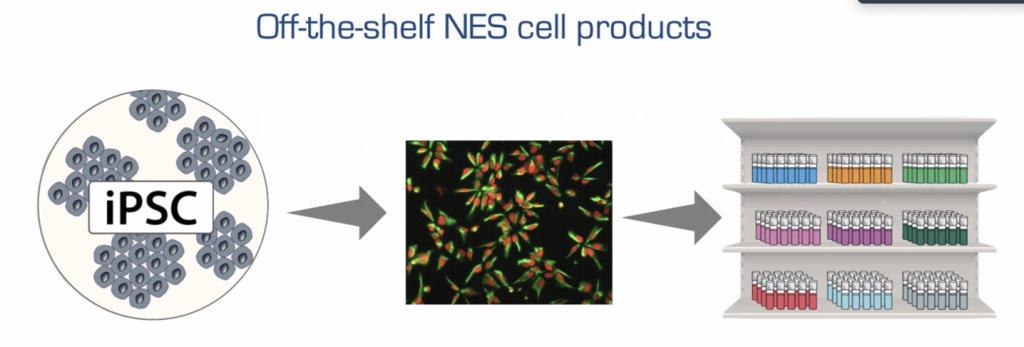 There are many ways the regenerative potential of cell transplantation therapy can be optimized to pave the route to the clinic. They include, but are not limited to, deciding which cells to transplant and looking at whether we should use fresh cells or cryopreserved cells.
There are many ways the regenerative potential of cell transplantation therapy can be optimized to pave the route to the clinic. They include, but are not limited to, deciding which cells to transplant and looking at whether we should use fresh cells or cryopreserved cells.
For example, there are different types of neural stem cells in the human brain. Very early in development (embryonic weeks 6-8), there are neuroepithelial stem (NES) cells, and they are responsible for building up the brain. Later, in the fetal brain (from embryonic weeks 10-12 and forward), the neural stem cells transform into a radial glial (RG) type of stem cell.
From her post-doc work, Dr. Anna Falk helped to distinguish these two human fetal-derived stem cells. She showed that they have different cell markers, that NES cells take a shorter time to proliferate compared to RGs, and that they have different differentiation profiles. (NES cells differentiate into 80-90 per cent neurons and 10-20 per cent glia cells, and RGs differentiate into 50 per cent neurons and 50 per cent glia.)
Dr. Anna Falk is now a Professor at Lund University and is affiliated with Karolinska Institutet in Sweden. She is also the Chief Scientific Officer of CCRM Nordic. In case you missed it, at last year’s Medicine by Design Symposium (December 9, 2024), she discussed one of her lab’s more current research endeavours: optimizing the storage, maintenance and manufacturing of transplantable induced pluripotent stem cell (iPSC)-derived NES cells.
In 2012, Dr. Falk and her collaborators showed that iPSC-derived NES cells do a better job in promoting recovery than fetal-derived RG cells after transplantation into mice with one type of spinal cord injury (SCI). In 2021, this experiment was repeated in rats with a different type of SCI in which there is cyst formation in the spinal cord. The research showed that NES cells shrink the cyst more than RG cells. For the rats that received no transplantation, the cyst only grew larger. Despite being stem cells, NES cells do not cause tumours once transplanted. For pre-clinical stroke models, it turns out that NES-derived cortical progenitors differentiate into mature cortical neurons post-transplantation and also do a good job in rescuing function.
In these studies, they transplanted fresh NES cells (cells were transplanted immediately after their production in the lab). But the results using fresh cells led to some transplants being really good and some that weren’t. A really good transplant is one where both the cyst length and volume shrank significantly, and not-so-good transplant outcomes were indicated by a cyst that typically remained around the same length and volume as it did pre-transplantation.
Variations in transplantation outcomes might be due to limitations in conducting different kinds of quality testing before transplantation when using fresh cells, as these tests may require lengthy procedures. A feasible quality test that can be done within a reasonable time on fresh cells immediately before transplantation is one that counts how many cells are alive in the sample. Cells pass quality control if there are more than 80 per cent live cells. There are other quality tests that can only be done when cells are no longer alive and take a certain amount of time that wouldn’t be feasible if transplanting fresh cells. One such test is immunostaining to test for differentiation potential of the cells. This procedure often takes a couple of days to complete.
The researchers wanted to know how frozen-down, then thawed batches of NES cells would perform after transplantation. They knew that one way to create consistency between cell doses (and therefore outcomes) is to freeze down many ready-to-use batches of cells from the same starting source of fresh NES cells. Equal quantities of one NES cell source are distributed into hundreds of vials (doses) and then frozen. Therefore, each separate vial will have a known number of cells and cell quality will be similar between the vials. A subset of these vials can be easily set aside just before freezing to run more tests for identity, purity, genome integrity, quality and cell count, while the frozen-down cells sit in a cryopreserved state for later use. Quality control is improved with the cryopreservation process because from these additional quantification tests, you can decide if you have a good batch or not.
In terms of their effectiveness in transplantation outcomes, frozen and then thawed NES cells shrank the cyst by 84.4 per cent. Compare that to the previous transplantation study, in which the transplanted fresh NES cells shrank the cyst by 46.9 per cent. Not only that, but all transplants turned out well and were very similar. The identity, differentiation profile and viability between frozen and then thawed NES cells were the same as fresh NES cells. However, fresh NES cells could not be quality tested as much as frozen doses of cells. This result was also shown to be sustained for at least two years with minimal batch-to-batch variability.
The first part of these experiments was done using Good Manufacturing Practices (GMP), meaning there are strict procedures to follow: media and factors must be free of animal products and anything unknown; scientists wear sterile space-like suits in temperature-controlled clean rooms; and two operators are always present for every experiment – just to name a few. This is a significant feat toward being able to translate their pre-clinical results to the clinic, considering all in-human clinical trials must be GMP-compliant.
Although no loss of quality was seen between fresh and cryopreserved undifferentiated NES cells, the downside is that the more differentiated cells are, the more sensitive they are to cryopreservation. It will be more difficult to transplant cryopreserved mature neuronal cell types for cell therapy.
Nonetheless, Dr. Falk’s team demonstrated that if you are using undifferentiated NES cells for transplantation, stored, ready-to-go, precise cryopreserved NES cells are the way to the future. This type of batched storage ultimately has the potential to create ease-of-access when it comes to getting it to SCI patients in a timely manner.
Referring to the difference between RGs and NES cells, Dr. Falk said: “Neural stem cells come in different flavours.” In addition, there are so many different sources of stem cells that have been undergoing clinical trials for transplantation. For example, BlueRock Therapeutics uses embryonic stem cells to create dopamine neurons, and it’s these dopamine progenitors that get implanted into Parkinson’s patients as part of these clinical trials specifically.
Mesenchymal stem cells, which are not neural, have been used in clinical trials to treat neurodegenerative diseases like Alzheimer’s, ALS and Parkinson’s disease. Some pre-clinical trials are looking into creating spheres (clusters of neural stem cells) for implantation. The Okano lab uses NES cells, but instead of creating single cells, they create neurospheres. Each sphere contains 5,000-10,000 cells, which means that it might be more difficult to count the precise numbers of cells going into each transplanted dose, and they are implanted into pre-clinical SCI models. As it turns out, they are also doing a clinical trial using these neurospheres (registry # in Japan: jRCTa031190228).
Some of the reasons why different stem cell sources are being used by different labs can be attributed to how long the cells have been around. Embryonic stem cells have been around longer than iPSCs (1998 versus 2006). Also, people use whatever they have access to. For example, the cell lines BlueRock uses are well characterized, have been around a long time, and have undergone many successful tests already.
Having different companies use different cell types adds some competition and room for learning, which should foster the most optimal outcomes. Only time will tell. At ISSCR 2024 in Copenhagen, Melissa Carpenter revealed that by the end of 2024, more than 1,200 patients had undergone transplantation of human pluripotent stem cell-derived cells across a total of 116 approved clinical trials worldwide.
For more information on individual trials, different diseases that are being treated, and the hurdles we’ve overcome to get to this point, I recommend checking out this newly released review article published in January 2025. It is incredible to see the big picture. The Okano lab’s iPSC-derived NES cells have been approved for clinical trials in SCI (registry # in Japan: jRCTa031190228) – and these are with fresh cells. Perhaps one day we will also have off-the-shelf cryopreserved stem cells for consistent therapeutic transplantation outcomes.
Advancing women in STEM starts with investing in girls (#IDWGS2025) 11 Feb 4:00 AM (2 months ago)
CCRM’s team of women engineers in 2015 for the #ILookLikeAnEngineer campaign, coincidentally the inaugural year of IDWGS. L-R Fernanda Masri PhD, Emily Titus PhD, May MacIntosh, Camila Londono PhD, Joanna Fromstein PEng., Lesley Chan PhD, and Hayley Christian.
2025 marks the tenth anniversary of the UN General Assembly’s declaration of February 11 as the International Day of Women and Girls in Science (IDWGS). UNESCO has named this year’s theme “Unpacking STEM Careers: Her Voice in Science.” This made me reflect on the persistent gender gap in the science, technology, engineering and mathematics (STEM) fields, and the fact that women are less likely to pursue STEM careers because, as girls, their STEM education is not prioritized or supported. After all, there’s a reason IDWGS specifically names girls: inspiring generations of women to launch successful careers in STEM starts by reaching them when they are young and providing equitable access to STEM education and opportunities.
Statistics Canada observed that female high school graduates are 29.8 per cent less likely than their male counterparts to choose a post-secondary STEM education, and 36.4 per cent less likely to pursue STEM bachelor’s degrees in particular. It follows, then, that women constitute less than a quarter of the people employed in these fields.
What are some reasons for this? There are many. One explanation is that women may decide against pursuing STEM careers because they seem incompatible to other parts of life, including motherhood. The expectation to work long, odd hours in a lab, for example, may not align with childcare responsibilities. However, although these barriers explain why working women of working age may not want STEM jobs, they don’t account for girls not choosing this career path.
To ensure generations of women to come have access and representation in these fields, the interest and involvement must be fostered in girls throughout elementary and secondary education. Below, I’ve laid out some ways that educators, parents and anybody passionate about getting girls interested in STEM can do their part.
- Getting girls involved in STEM extracurriculars
In my role as Communications Coordinator at CCRM, I’ve worked closely with Visions of Science, a non-profit organization dedicated to providing equitable access and representation in STEM education, and opportunities for Black and racialized youth in low-income communities across the Greater Toronto Area. The organization isn’t dedicated specifically to providing girls with access to STEM, but they’re tackling disadvantage and inequality from other fronts, such as race and income barriers.
Months ago, I attended a Visions of Science showcase event, where students in grades 5-11 presented science experiments that they worked on in their summer camp programs. In the presentations from younger students, what struck me was the creativity and originality necessary to come up with and execute the experiments (my favourite: “Does swallowed gum stay in your stomach for seven years?” accompanied by a 3-D diagram of the digestive tract). This is an important point to emphasize when presenting STEM to girls (and boys): it isn’t all analytical, all the time. There’s a place for creativity, originality and fun.
The older students, who were in high school, had the opportunity to take on summer internships at institutions like the University of Toronto and York University. What better way to get youth—male or female—excited about these career possibilities than having them intern in state-of-the-art facilities, with some of the world’s top professors and researchers? What it comes down to is opportunity and exposure: the opportunity to work in these spaces will instill in girls the confidence to follow STEM into post-secondary education.
There are other organizations focused on providing girls, specifically, with STEM access. The Girl Guides of Canada, for example, launched a Girls In STEM program. If STEM extra-curricular programs are normalized to the same degree as sports or arts, students’ perception of the fields will shift from purely academic to genuine interest.
- Giving girls female STEM role models
How can we expect girls to follow STEM post-secondary education and careers when, from the outside-in, they still seem like a boys’ club? There are a plethora of female scientists, engineers and mathematicians who have changed our world, and by teaching girls about these women, they’ll see that they can go on to do the same. Take Mary Jackson (the first African American female engineer at NASA), Katherine Johnson and Dorothy Vaughan, the NASA employees who inspired the movie “Hidden Figures.” The movie was about their work and how it helped make history in the 1960s during the United States’ space race with the Soviet Union.
Perhaps girls would be interested to learn about Marie Curie, the first woman to win a Nobel Prize, or Ada Lovelace, the first person—man or woman—to write a computer program. We don’t even need to look to history for great female role models: we can look to Dr. Kizzmekia Corbett, who was at the forefront of developing the Moderna Covid-19 vaccine. The more that girls see examples of women pursuing STEM, breaking barriers and societal expectations, early in their education, the more likely they are to view STEM as a feasible option.
- Being a STEM mentor
While public and historical figures can light a spark in girls’ interest in STEM, consistent and personal mentorship will nurture it into a viable career path. If you’re a woman working in STEM, consider reaching out to STEM groups to see how you can get involved. Find some suggestions below.
Visions of Science (Toronto)
Canadian Association for Girls In Science (CAGIS) (Canada)
Society for Canadian Women in Science & Technology (Canada)
Girls Who Code (international)
Million Women Mentors (international)
500 Women Scientists (international)
Women working in STEM can also encourage girls’ interest in the fields by being visible. In the featured picture above, CCRM’s female engineers participated in the #ILookLikeAnEngineer campaign to raise awareness of gender stereotypes in engineering. Read the post from 2015 here and note that CCRM’s workforce was much smaller a decade ago.
By investing in STEM education for girls early on in their lives, the returns are immeasurable, as evidenced by female scientists like Dr. Corbett. On this International Day of Women and Girls in Science, let’s commit to doing our part to get girls interested, involved and invested in STEM.





